The Monomer Of A Protein Is
Muz Play
Mar 22, 2025 · 6 min read
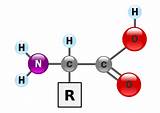
Table of Contents
The Monomer of a Protein Is: An In-Depth Look at Amino Acids and Peptide Bonds
Proteins are the workhorses of the cell, carrying out a vast array of functions crucial for life. From catalyzing biochemical reactions to providing structural support, proteins' diverse roles stem from their unique structures, which are ultimately determined by their fundamental building blocks: amino acids. Therefore, the answer to the question, "The monomer of a protein is..." is unequivocally an amino acid.
Understanding Amino Acids: The Building Blocks of Proteins
Amino acids are organic molecules containing a central carbon atom (the α-carbon) bonded to four different chemical groups:
- An amino group (-NH₂): This group is basic and carries a positive charge at physiological pH.
- A carboxyl group (-COOH): This group is acidic and carries a negative charge at physiological pH.
- A hydrogen atom (-H): A simple hydrogen atom.
- A side chain (R-group): This is the variable group that distinguishes one amino acid from another. The R-group can be anything from a simple hydrogen atom (as in glycine) to a complex aromatic ring structure (as in tryptophan).
It's this R-group that dictates the unique properties of each amino acid, influencing its size, shape, charge, polarity, and reactivity. These properties, in turn, determine how amino acids interact with each other and with their surrounding environment, ultimately shaping the protein's three-dimensional structure and function.
The 20 Standard Amino Acids
There are 20 standard amino acids commonly found in proteins. These amino acids are genetically encoded and used by ribosomes during protein synthesis. They are often categorized based on their R-group properties:
- Nonpolar, aliphatic amino acids: These amino acids have hydrophobic (water-repelling) side chains, often consisting of hydrocarbon chains. Examples include glycine, alanine, valine, leucine, isoleucine, and methionine.
- Aromatic amino acids: These amino acids possess aromatic rings in their side chains, contributing to their hydrophobic nature and ability to absorb ultraviolet light. Examples include phenylalanine, tyrosine, and tryptophan.
- Polar, uncharged amino acids: These amino acids have hydrophilic (water-attracting) side chains that can form hydrogen bonds. Examples include serine, threonine, cysteine, asparagine, and glutamine.
- Positively charged (basic) amino acids: These amino acids have side chains with a positive charge at physiological pH. Examples include lysine, arginine, and histidine.
- Negatively charged (acidic) amino acids: These amino acids have side chains with a negative charge at physiological pH. Examples include aspartic acid and glutamic acid.
Peptide Bonds: Linking Amino Acids Together
Individual amino acids are joined together to form proteins through a process called peptide bond formation. This involves a dehydration reaction, where the carboxyl group of one amino acid reacts with the amino group of another amino acid, releasing a water molecule (H₂O) and forming a covalent bond between the α-carbon of one amino acid and the nitrogen atom of the next. This covalent bond is known as a peptide bond.
The resulting chain of amino acids is called a polypeptide. A protein can consist of one or more polypeptide chains, folded into a specific three-dimensional structure. The sequence of amino acids in a polypeptide chain is called the primary structure of the protein. This primary structure dictates the higher-order structures (secondary, tertiary, and quaternary) which determine the protein's overall function.
The Directionality of Polypeptides
Polypeptide chains have a defined directionality, with an N-terminus (amino terminus) and a C-terminus (carboxyl terminus). The N-terminus corresponds to the free amino group of the first amino acid in the chain, while the C-terminus corresponds to the free carboxyl group of the last amino acid. Proteins are always synthesized from the N-terminus to the C-terminus.
Beyond the 20 Standard Amino Acids: Modified and Uncommon Amino Acids
While the 20 standard amino acids form the foundation of protein structure, it's important to note that other amino acids can be incorporated into proteins through post-translational modifications. These modifications can alter the properties of the amino acid and thus affect the protein's function. Examples include:
- Phosphorylation: The addition of a phosphate group to serine, threonine, or tyrosine residues. This is a common mechanism for regulating protein activity.
- Glycosylation: The attachment of carbohydrate molecules to asparagine, serine, or threonine residues. This is crucial for protein folding, stability, and cell signaling.
- Hydroxylation: The addition of a hydroxyl group to proline or lysine residues. This modification is essential for collagen stability.
Additionally, some proteins contain non-standard amino acids, which are not directly incorporated during translation but are modified after protein synthesis or are derived from standard amino acids through enzymatic reactions. These non-standard amino acids can play important roles in protein structure and function.
The Importance of Amino Acid Sequence and Protein Structure
The precise sequence of amino acids in a polypeptide chain (primary structure) is critical for determining the protein's three-dimensional structure and, consequently, its function. This sequence dictates how the polypeptide chain will fold into its functional conformation.
Higher-Order Protein Structures
- Secondary structure: refers to local folding patterns within the polypeptide chain, such as alpha-helices and beta-sheets. These structures are stabilized by hydrogen bonds between amino acid backbone atoms.
- Tertiary structure: refers to the overall three-dimensional arrangement of a single polypeptide chain. This structure is stabilized by various interactions between amino acid side chains, including hydrogen bonds, ionic bonds, disulfide bridges, and hydrophobic interactions.
- Quaternary structure: refers to the arrangement of multiple polypeptide chains (subunits) to form a functional protein complex. This structure is also stabilized by various interactions between the subunits.
Any alteration in the amino acid sequence, such as a single amino acid substitution, can significantly impact the protein's structure and function. This is evident in genetic diseases caused by mutations that alter the amino acid sequence of a crucial protein.
Protein Functions: A Diverse Repertoire
The diversity of protein functions arises from the immense variability in amino acid sequences and the resulting three-dimensional structures. Some key protein functions include:
- Enzymes: Catalyze biochemical reactions, speeding up metabolic processes.
- Structural proteins: Provide structural support and mechanical strength to cells and tissues (e.g., collagen, keratin).
- Transport proteins: Carry molecules across cell membranes or throughout the body (e.g., hemoglobin).
- Motor proteins: Generate movement within cells or organisms (e.g., myosin, kinesin).
- Hormones: Act as chemical messengers, regulating various physiological processes.
- Receptor proteins: Bind to specific molecules and initiate cellular responses.
- Antibodies: Part of the immune system, recognizing and binding to foreign antigens.
Conclusion: The Central Role of Amino Acids in Protein Biology
In conclusion, the monomer of a protein is the amino acid. The 20 standard amino acids, each with unique properties dictated by their side chains, combine through peptide bonds to form polypeptide chains. The precise sequence of amino acids determines the protein's primary structure, which in turn dictates its higher-order structures and ultimately its biological function. Understanding the properties of amino acids and the principles of protein structure is fundamental to comprehending the diverse roles proteins play in all aspects of life. From the simplest enzymes to the most complex cellular machinery, proteins are the building blocks and workhorses of life, and their remarkable diversity stems directly from the fundamental unit: the amino acid. The study of amino acids and their interactions remains a cornerstone of modern biochemistry and molecular biology, with ongoing research continually revealing new insights into their roles in health and disease.
Latest Posts
Latest Posts
-
Cells The Basic Unit Of Life
Mar 22, 2025
-
Atomic Number Equals The Number Of
Mar 22, 2025
-
Solids To Gases Row Or Column
Mar 22, 2025
-
What Color Is The Animal Cell
Mar 22, 2025
-
Is Silver A Metal Or Nonmetal Or Metalloid
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about The Monomer Of A Protein Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
