Atomic Number Equals The Number Of
Muz Play
Mar 22, 2025 · 6 min read
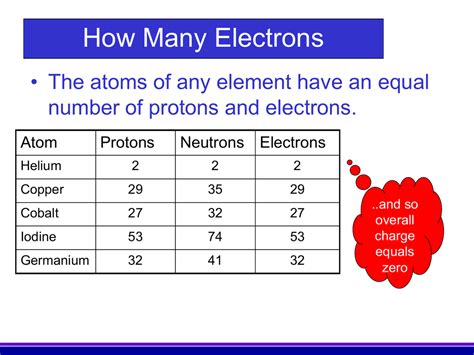
Table of Contents
Atomic Number Equals the Number of Protons: A Deep Dive into the Foundation of Chemistry
The seemingly simple statement, "atomic number equals the number of protons," underpins the entire field of chemistry. It's a fundamental concept that dictates an element's identity, its properties, and its behavior in chemical reactions. This article will delve into this core principle, exploring its implications for understanding the periodic table, isotopic variations, and the broader world of atomic structure.
What is Atomic Number?
The atomic number of an element is a crucial identifying characteristic, representing the number of protons found in the nucleus of a single atom of that element. It's denoted by the symbol Z. This number is unique to each element and is what distinguishes hydrogen (Z=1) from helium (Z=2), carbon (Z=6), uranium (Z=92), and all other elements on the periodic table. It's not simply a count; it's the fundamental identifier. Think of it as an element's atomic fingerprint.
The Significance of Protons
Protons are positively charged subatomic particles residing within the atom's nucleus. They possess a mass approximately 1836 times greater than that of an electron. Crucially, the number of protons determines the element's identity. Changing the number of protons fundamentally alters the element itself. Adding a proton transforms an atom of one element into an atom of the next element on the periodic table.
Neutrons and Electrons: The Other Subatomic Players
While protons define the element, neutrons and electrons also play vital roles in atomic structure. Neutrons, also located in the nucleus, are neutral particles with a mass similar to protons. They contribute to the atom's mass but not its charge. Electrons, negatively charged particles orbiting the nucleus, are far lighter than protons and neutrons. Their number usually equals the number of protons in a neutral atom, ensuring a balanced charge.
The Periodic Table: A Testament to Atomic Number
The periodic table, a cornerstone of chemistry, is organized primarily by atomic number. Elements are arranged sequentially according to increasing atomic number, reflecting their fundamental properties and recurring trends in chemical behavior. This arrangement is not arbitrary; it arises directly from the underlying structure dictated by the number of protons. The table's rows (periods) and columns (groups) reflect the electron configurations of the atoms, which are, in turn, determined by the number of protons.
Periodic Trends and Atomic Number
Many periodic trends, such as electronegativity, ionization energy, and atomic radius, are directly related to atomic number. As you move across a period (left to right), the atomic number increases, leading to an increase in the number of protons and electrons. This results in a stronger nuclear charge, attracting the outermost electrons more tightly and influencing the element's reactivity.
Moving down a group (top to bottom), the atomic number also increases, but the added electrons occupy higher energy levels, further from the nucleus. This increases the atomic radius and generally reduces the element's electronegativity. These trends are predictable and directly linked to the fundamental concept of atomic number.
Isotopes: Variations in Neutron Number
While atomic number remains constant for a given element, the number of neutrons can vary. Isotopes are atoms of the same element (same atomic number) that have different numbers of neutrons. This means they have the same number of protons but different mass numbers (the sum of protons and neutrons).
Isotopic Abundance and Atomic Mass
Different isotopes of an element exist in varying abundances in nature. The atomic mass of an element listed on the periodic table is a weighted average of the masses of its naturally occurring isotopes, reflecting their relative abundances. For example, carbon has two main isotopes: carbon-12 (⁶¹²C) and carbon-13 (⁶¹³C). The atomic mass of carbon is approximately 12.01 amu, reflecting the higher abundance of carbon-12.
Applications of Isotopes
Isotopes have various applications in different fields:
- Carbon-14 dating: Used to determine the age of organic materials.
- Medical imaging: Isotopes like technetium-99m are used in various medical imaging techniques.
- Nuclear medicine: Isotopes are employed in radiation therapy for cancer treatment.
- Scientific research: Isotopes are crucial tools in various scientific investigations, offering insights into chemical reactions and biological processes.
Beyond the Basics: Implications for Nuclear Chemistry and Physics
The atomic number, while seemingly a simple concept, has profound implications in nuclear chemistry and physics. Nuclear reactions involve changes in the nucleus, often altering the number of protons and neutrons.
Nuclear Fusion and Fission
Nuclear fusion involves the combining of atomic nuclei, while nuclear fission involves the splitting of an atomic nucleus. Both processes alter the atomic number, resulting in the formation of new elements. Understanding atomic number is fundamental to comprehending these energy-releasing processes, which have profound implications for energy production and weapons technology.
Nuclear Stability and Radioactive Decay
The stability of an atom's nucleus is influenced by the ratio of protons to neutrons. Atoms with unstable nuclei undergo radioactive decay, emitting particles or energy to achieve a more stable configuration. This decay process often involves changes in the atomic number, transforming one element into another. Understanding these decay processes is crucial in various fields, including nuclear medicine and geology.
Conclusion: The Foundation of Chemical Understanding
The statement "atomic number equals the number of protons" is far more than a simple definition. It represents the foundational principle upon which our understanding of chemistry is built. From the organization of the periodic table to the behavior of isotopes and the intricacies of nuclear reactions, the atomic number provides a crucial framework for comprehending the structure and properties of matter. Its simplicity belies its profound implications, underscoring its central role in the vast and fascinating world of atoms and molecules. Further exploration of this fundamental concept opens doors to deeper understanding of the universe and the matter that composes it. The seemingly simple number Z unlocks a wealth of knowledge, highlighting the power of fundamental principles in scientific discovery. It's a cornerstone of modern science, a key that unlocks a universe of chemical understanding. By understanding this basic yet powerful concept, we can unlock the secrets of the elements and their interactions, enabling advances in countless fields, from medicine to materials science and beyond. The study of atomic number is an ongoing journey of discovery, continually revealing new insights into the nature of matter and the universe itself. This simple concept holds the key to a vast and complex world, awaiting further exploration and understanding. The implications of this simple statement are far-reaching, extending far beyond the classroom and into the frontiers of scientific research and technological advancement. The enduring importance of this fundamental principle should not be underestimated.
Latest Posts
Latest Posts
-
Chemical Equilibrium Le Chateliers Principle Lab Report
Mar 23, 2025
-
Foundations Of Maternal Newborn And Womens Health Nursing
Mar 23, 2025
-
Pie Chart Of The Cell Cycle
Mar 23, 2025
-
A Que Temperatura Se Congela El Agua En Grados Fahrenheit
Mar 23, 2025
-
Blood Flow Through The Capillary Beds Is Regulated By
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Atomic Number Equals The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
