Is Silver A Metal Or Nonmetal Or Metalloid
Muz Play
Mar 22, 2025 · 5 min read
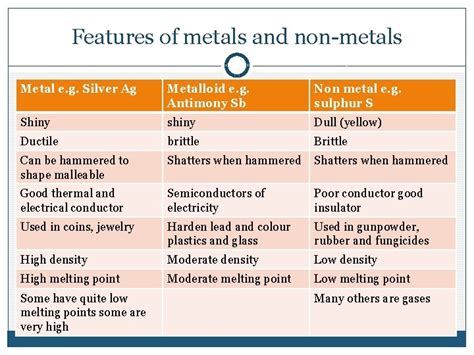
Table of Contents
Is Silver a Metal, Nonmetal, or Metalloid? A Deep Dive into Silver's Properties
Silver, a lustrous white metal known for its exceptional conductivity and malleability, holds a prominent place in various industries, from electronics to jewelry. But where does it sit on the periodic table's classification of elements? Is silver a metal, nonmetal, or metalloid? The answer is clear: silver is a metal. This article delves deep into silver's properties, exploring why it unequivocally falls into the metal category, and examining its unique characteristics that make it so valuable.
Understanding the Classification of Elements
Before we definitively classify silver, let's review the fundamental differences between metals, nonmetals, and metalloids. These classifications are based on elements' physical and chemical properties:
Metals
Metals are generally characterized by:
- High electrical conductivity: They readily conduct electricity.
- High thermal conductivity: They efficiently transfer heat.
- Malleability and ductility: They can be hammered into sheets (malleability) and drawn into wires (ductility).
- Metallic luster: They possess a shiny appearance.
- High density: They are generally denser than nonmetals.
- Tend to lose electrons: They readily form positive ions (cations) in chemical reactions.
Nonmetals
Nonmetals, in contrast, typically exhibit:
- Poor electrical conductivity: They are generally poor conductors of electricity.
- Poor thermal conductivity: They are inefficient at transferring heat.
- Brittle: They tend to be brittle and easily shatter.
- Lack of metallic luster: They often lack a shiny appearance.
- Low density: They are generally less dense than metals.
- Tend to gain electrons: They readily form negative ions (anions) in chemical reactions.
Metalloids (Semimetals)
Metalloids occupy an intermediate position between metals and nonmetals, displaying properties of both:
- Intermediate electrical conductivity: Their conductivity is between that of metals and nonmetals, often varying with temperature or other conditions. This property makes them useful in semiconductors.
- Intermediate thermal conductivity: Their heat transfer capabilities lie between metals and nonmetals.
- Variable physical properties: Their properties can be more unpredictable and vary depending on the specific metalloid and its conditions.
Silver's Definitive Metallic Properties
Now, let's examine silver's properties to definitively confirm its classification as a metal. Silver exhibits all the hallmark characteristics of a metal:
1. Exceptional Electrical Conductivity
Silver boasts the highest electrical conductivity of any element. This extraordinary property is why it's crucial in electronics, used in electrical contacts, circuits, and other applications requiring minimal electrical resistance. This unparalleled conductivity strongly points to its metallic nature.
2. Excellent Thermal Conductivity
Similarly, silver possesses exceptional thermal conductivity. It efficiently transfers heat, making it suitable for applications requiring efficient heat dissipation, such as heat sinks in electronics or specialized heat exchangers. This high thermal conductivity further reinforces its metallic classification.
3. High Malleability and Ductility
Silver is highly malleable, meaning it can be easily hammered or rolled into thin sheets. It's also highly ductile, meaning it can be drawn into wires without breaking. These properties are quintessential characteristics of metals and are exploited extensively in silver's applications, from jewelry making to the creation of intricate electrical components.
4. Characteristic Metallic Luster
Silver possesses a bright, lustrous white appearance, a classic hallmark of metals. This inherent shine, combined with its ability to be polished to a high degree, makes it highly desirable for decorative purposes, including jewelry and silverware.
5. Relatively High Density
Silver has a relatively high density compared to nonmetals. While not the densest metal, its density clearly falls within the range typical of metals.
6. Chemical Behavior: Electron Donation
Silver's chemical behavior also aligns with that of metals. It tends to lose electrons readily, forming positive ions (Ag⁺) in chemical reactions. This electron donation is a defining characteristic of metals, further solidifying its classification.
Silver's Applications: A Testament to its Metallic Nature
The wide range of applications for silver is a direct result of its unique metallic properties. Its versatility highlights its suitability for various technological and industrial processes:
- Electronics: Silver's unparalleled electrical conductivity makes it invaluable in microelectronics, printed circuit boards (PCBs), and other electronic components where minimal resistance is crucial.
- Photography: Silver halides have historically been used in photographic film and paper due to their light sensitivity.
- Catalysis: Silver is used as a catalyst in various chemical reactions.
- Jewelry and Ornamental Items: Silver's beauty, malleability, and resistance to corrosion have made it a highly prized material for jewelry and decorative items for centuries.
- Medicine: Silver nanoparticles have demonstrated antimicrobial properties, leading to their use in wound dressings and other medical applications.
- Solar Cells: Silver's high conductivity makes it an excellent choice for electrode materials in solar cells.
- Mirrors: Silver's reflectivity makes it ideal for coating mirrors, enhancing their reflective capabilities.
Addressing Potential Confusion: Silver's Unique Reactions
While silver clearly exhibits metallic characteristics, some of its chemical reactions might seem atypical at first glance. For example, silver can tarnish, forming a layer of silver sulfide (Ag₂S) on exposure to air containing sulfur compounds. This tarnishing isn't evidence against silver's metallic nature; it's a chemical reaction characteristic of many metals, particularly those with lower reactivity. The tarnishing process doesn't negate its fundamentally metallic properties.
Furthermore, silver’s relatively low reactivity compared to other alkali metals might lead some to question its metallic classification. However, reactivity is just one characteristic among many. Silver's other definitive metallic properties—its conductivity, malleability, ductility, and luster—far outweigh its relatively low reactivity. It's crucial to consider the full spectrum of properties when classifying an element.
Conclusion: Silver is Undeniably a Metal
In conclusion, the overwhelming evidence points to the fact that silver is a metal. Its exceptional electrical and thermal conductivity, high malleability and ductility, metallic luster, relatively high density, and tendency to lose electrons in chemical reactions all strongly support this classification. While some of its chemical behavior might initially appear unconventional, a holistic consideration of its properties confirms its place among the metals on the periodic table. Silver’s extensive applications across numerous industries further demonstrate the immense value of its inherent metallic characteristics. The question "Is silver a metal, nonmetal, or metalloid?" has a clear and definitive answer: silver is a metal, and its unique combination of properties makes it a remarkably valuable element.
Latest Posts
Latest Posts
-
Find Determinant By Row Reduction To Echelon Form
Mar 23, 2025
-
Duvalls Developmental Stages Of The Family
Mar 23, 2025
-
The Graph Shows The Oxygen Binding Curves For Myoglobin And Hemoglobin
Mar 23, 2025
-
Liquids Gases And Solids Periodic Table
Mar 23, 2025
-
Fundamentals Of General Organic And Biological Chemistry Mcmurry
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Is Silver A Metal Or Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
