Liquids Gases And Solids Periodic Table
Muz Play
Mar 23, 2025 · 6 min read
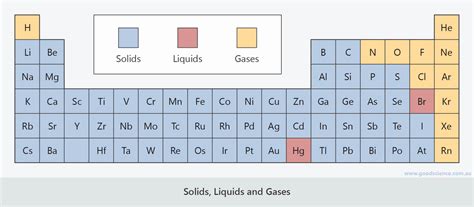
Table of Contents
Liquids, Gases, and Solids: A Periodic Table Perspective
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. While it doesn't directly categorize matter into its physical states (solid, liquid, gas), it provides invaluable insight into the relationship between an element's atomic characteristics and the state it adopts under specific conditions. Understanding this relationship is crucial for comprehending the behavior of matter in various applications, from material science to environmental studies. This article delves into the connection between the periodic table, the properties of elements, and the states of matter – solids, liquids, and gases.
The Role of Intermolecular Forces
The physical state of a substance – solid, liquid, or gas – is primarily determined by the strength of intermolecular forces between its constituent particles (atoms, molecules, or ions). These forces are not as strong as the intramolecular forces (bonds) within a molecule, but they significantly influence the behavior of matter in bulk. The periodic table provides clues to predict the strength of these forces.
1. London Dispersion Forces (LDFs): The Universal Force
All atoms and molecules experience London Dispersion Forces (LDFs), also known as van der Waals forces. These are weak, temporary attractions arising from instantaneous fluctuations in electron distribution. Larger atoms and molecules with more electrons exhibit stronger LDFs because of their greater polarizability (ease of electron cloud distortion). Consequently, elements located further down and to the right of the periodic table (excluding noble gases) tend to have stronger LDFs. This explains why, for example, bromine (Br₂) is a liquid at room temperature, whereas chlorine (Cl₂) is a gas. Bromine has larger atoms and stronger LDFs, resulting in greater intermolecular attraction and a liquid state.
2. Dipole-Dipole Interactions: Polarity Matters
Molecules with polar bonds, possessing a permanent dipole moment (a separation of positive and negative charge), experience dipole-dipole interactions. These forces are stronger than LDFs. The polarity of a molecule is determined by the electronegativity difference between the constituent atoms. Electronegativity generally increases across a period and decreases down a group in the periodic table. Elements with high electronegativity attract electrons more strongly, leading to polar bonds and stronger dipole-dipole interactions. For instance, water (H₂O) is a liquid at room temperature due to strong dipole-dipole interactions between its polar molecules.
3. Hydrogen Bonding: A Special Case
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a nearby molecule. This type of bonding is responsible for the unusually high boiling point of water compared to other hydrides in its group (e.g., H₂S, H₂Se). The elements involved in hydrogen bonding are primarily located in the upper right corner of the periodic table. Hydrogen bonding significantly influences the properties of biological molecules like proteins and DNA.
4. Ionic Interactions: The Strongest of All
Ionic compounds, formed by the electrostatic attraction between oppositely charged ions, have strong intermolecular forces. The strength of these interactions depends on the magnitude of the charges and the distance between the ions. Ionic compounds generally exist as solids at room temperature due to these strong attractions. The position of elements on the periodic table indicates their tendency to form ions: metals (left side) tend to lose electrons and form positive ions, while nonmetals (right side) tend to gain electrons and form negative ions.
Predicting States of Matter from Periodic Trends
By considering the trends in electronegativity, atomic size, and the resultant intermolecular forces, we can make some predictions about the states of matter of elements and compounds.
-
Metals: Generally solid at room temperature due to strong metallic bonding, which involves a "sea" of delocalized electrons. Their melting points vary widely, depending on the strength of the metallic bonds. The alkali metals (Group 1) have relatively low melting points, while transition metals (d-block) often have high melting points.
-
Nonmetals: Their states vary significantly. Many exist as diatomic gases (e.g., H₂, O₂, N₂, F₂, Cl₂), while some are solids (e.g., carbon, sulfur, phosphorus). Their states are dictated by the types and strengths of intermolecular forces present.
-
Metalloids: Exhibit properties intermediate between metals and nonmetals. Some are solids at room temperature.
-
Noble Gases: Exist as monatomic gases at room temperature due to their very weak intermolecular forces (only LDFs). Their lack of reactivity stems from their full valence electron shells.
The Impact of Temperature and Pressure
While the periodic table helps us understand the inherent properties of elements that influence their states, it's crucial to remember that temperature and pressure play significant roles in determining the physical state of a substance. Increasing temperature generally increases the kinetic energy of particles, weakening intermolecular forces and favoring the liquid and gaseous states. Increasing pressure favors the condensed states (solid and liquid) by reducing the volume occupied by the particles and increasing the effectiveness of intermolecular forces. Phase diagrams visually represent the relationship between temperature, pressure, and the state of a substance.
Examples Across the Periodic Table
Let's consider specific examples to illustrate the link between the periodic table, elemental properties, and states of matter.
-
Group 17 (Halogens): Fluorine (F₂) and Chlorine (Cl₂) are gases, bromine (Br₂) is a liquid, and iodine (I₂) is a solid at room temperature. This trend reflects the increasing strength of LDFs as we move down the group due to larger atomic size and increased electron count.
-
Group 18 (Noble Gases): All are gases at room temperature due to weak intermolecular forces (only LDFs) and their reluctance to form chemical bonds. Their boiling points increase down the group due to stronger LDFs.
-
Group 14 (Carbon Group): Carbon exists as a solid (diamond, graphite), silicon and germanium are metalloid solids, tin is a metal (solid), and lead is also a metal (solid). The trend reflects the gradual change in bonding characteristics from covalent (carbon) to metallic bonding (tin and lead).
-
Transition Metals: The majority are hard, solid metals at room temperature with high melting points. This is due to their strong metallic bonding, which arises from the delocalized electrons in their d-orbitals.
Conclusion
The periodic table provides a framework for understanding the fundamental properties of elements, which in turn influence their behavior in different states of matter. While the table doesn't explicitly classify elements based on their physical states, it reveals crucial trends in atomic size, electronegativity, and bonding characteristics that dictate the strengths of intermolecular forces. These forces, along with the interplay of temperature and pressure, ultimately determine whether a substance exists as a solid, liquid, or gas. This understanding is fundamental across various scientific disciplines, from materials science and engineering to environmental chemistry and biochemistry. By linking periodic trends with intermolecular forces and phase transitions, we gain a deeper comprehension of the macroscopic world from the fundamental properties of its constituent elements.
Latest Posts
Latest Posts
-
An Ion Has Unequal Numbers Of
Mar 25, 2025
-
Calculate Average Atomic Mass Of Isotopes
Mar 25, 2025
-
Understanding Theoretical Actual And Percent Yield
Mar 25, 2025
-
Are The Most Organized State Of Matter Responses
Mar 25, 2025
-
Which Alkane Is The Isomer Of Butane Called 2 Methylpropane
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Liquids Gases And Solids Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
