Which Alkane Is The Isomer Of Butane Called 2 Methylpropane
Muz Play
Mar 25, 2025 · 5 min read
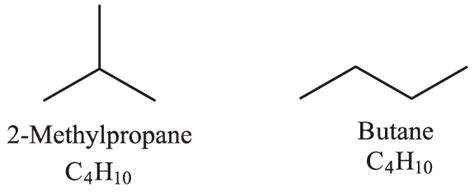
Table of Contents
Which Alkane Is the Isomer of Butane Called 2-Methylpropane?
Understanding isomers is crucial in organic chemistry. Isomers are molecules that share the same molecular formula but differ in their structural arrangement. This seemingly small difference can lead to vastly different physical and chemical properties. This article delves deep into the isomeric relationship between butane and 2-methylpropane, exploring their structures, properties, and the broader implications of isomerism in the world of alkanes.
What are Alkanes and Isomers?
Before we dive into the specifics of butane and 2-methylpropane, let's establish a firm foundation in the basics. Alkanes are saturated hydrocarbons – meaning they contain only single carbon-carbon bonds and are composed solely of carbon and hydrogen atoms. They are the simplest class of organic compounds, forming the basis for many more complex molecules. The general formula for alkanes is C<sub>n</sub>H<sub>2n+2</sub>, where 'n' represents the number of carbon atoms.
Isomers, as mentioned earlier, are molecules with the same molecular formula but different structural formulas. This difference in arrangement significantly impacts their properties. There are several types of isomerism, including structural isomerism (also known as constitutional isomerism) and stereoisomerism. In the case of butane and 2-methylpropane, we're dealing with structural isomers. These isomers differ in the way their atoms are connected, leading to variations in their branching patterns.
Butane: The Straight-Chain Alkane
Butane, with a molecular formula of C<sub>4</sub>H<sub>10</sub>, is a simple alkane with four carbon atoms arranged in a straight chain. Its structural formula can be represented as:
CH3-CH2-CH2-CH3
This linear structure gives butane specific properties. It is a gas at room temperature, relatively unreactive, and has a relatively low boiling point compared to its branched isomers. Its properties are largely dictated by the relatively strong London dispersion forces between its molecules. The longer, straighter chain allows for more surface contact and stronger intermolecular interactions.
Properties of Butane
- Molecular Formula: C₄H₁₀
- Boiling Point: -0.5 °C
- Melting Point: -138.3 °C
- State at Room Temperature: Gas
- Reactivity: Relatively unreactive (undergoes combustion and halogenation)
2-Methylpropane: The Branched-Chain Isomer
2-Methylpropane, also known as isobutane, is a structural isomer of butane. It also has the molecular formula C<sub>4</sub>H<sub>10</sub>, but its carbon atoms are arranged differently. Instead of a straight chain, 2-methylpropane has a branched structure:
CH3
|
CH3-C-CH3
|
CH3
This branching significantly alters its properties compared to butane. The compact, branched structure minimizes surface area contact between molecules, resulting in weaker intermolecular forces. This directly affects its physical properties.
Properties of 2-Methylpropane
- Molecular Formula: C₄H₁₀
- Boiling Point: -11.7 °C
- Melting Point: -159.6 °C
- State at Room Temperature: Gas
- Reactivity: Similar reactivity to butane, but reaction rates may differ slightly due to steric hindrance.
Comparing Butane and 2-Methylpropane: A Detailed Analysis
The key difference between butane and 2-methylpropane lies in their structural isomerism. This seemingly subtle difference leads to observable variations in several properties:
1. Boiling Point: 2-Methylpropane has a significantly higher boiling point (-11.7 °C) than butane (-0.5 °C). This is because the branched structure of 2-methylpropane reduces the surface area available for intermolecular interactions. The weaker London dispersion forces in 2-methylpropane require less energy to overcome, hence the lower boiling point.
2. Melting Point: Similarly, the melting point of 2-methylpropane (-159.6 °C) is lower than that of butane (-138.3 °C). The reduced surface area and weaker intermolecular forces also influence the melting point.
3. Density: While both are gases at room temperature, the density will vary slightly. The more compact structure of 2-methylpropane might lead to a slightly higher density, although the difference may be minimal.
4. Reactivity: Both butane and 2-methylpropane exhibit similar reactivity in combustion and halogenation reactions. However, the steric hindrance caused by the branching in 2-methylpropane can influence reaction rates. Reactions involving bulky reactants might proceed slower with 2-methylpropane due to this steric hindrance.
5. Solubility: Solubility in nonpolar solvents is generally similar for both isomers, given their nonpolar nature.
6. Spectral Properties: Different structural arrangements lead to distinct spectral fingerprints (IR, NMR, Mass Spec). These differences in spectral data can be used to identify and distinguish between butane and 2-methylpropane.
The Importance of Isomerism in Organic Chemistry
The isomerism exhibited by butane and 2-methylpropane is just one example of the widespread phenomenon of isomerism in organic chemistry. The existence of isomers highlights the importance of structural representation in understanding the properties and behavior of organic molecules. Small changes in structure can lead to dramatic variations in physical properties and, in some cases, chemical reactivity.
Understanding isomerism is crucial in several areas:
-
Petroleum Refining: Petroleum is a complex mixture of hydrocarbons, including many isomers. The properties of these isomers influence their suitability for different applications, such as gasoline or fuel oil. Isomerization processes are used in refineries to optimize fuel properties.
-
Pharmaceutical Industry: Isomers can have vastly different biological activities. In many drugs, only one specific isomer exhibits the desired therapeutic effect, while others may be inactive or even toxic. Therefore, precise isomeric control is essential in drug synthesis and formulation.
-
Polymer Chemistry: The arrangement of monomer units in polymers can significantly influence the properties of the resulting material. Isomerism plays a significant role in determining the mechanical, thermal, and other properties of polymers.
-
Food Science: The isomeric composition of fatty acids influences the nutritional value and physical properties of food products.
Conclusion: Beyond Butane and 2-Methylpropane
The isomeric relationship between butane and 2-methylpropane provides a clear and concise example of the importance of structural isomerism in organic chemistry. Their differing properties, despite sharing the same molecular formula, demonstrate how subtle changes in atomic arrangement can have profound effects on physical and, to a lesser extent, chemical properties. This fundamental concept extends far beyond these two simple alkanes, influencing the properties and applications of countless organic molecules across various fields, from petroleum refining to pharmaceuticals. The study of isomers is therefore not simply an academic exercise; it's a cornerstone of understanding the complex world of organic chemistry and its applications in the real world. Further exploration of other isomeric forms and their implications in various chemical applications would enhance a deeper understanding of this fundamental aspect of organic chemistry.
Latest Posts
Latest Posts
-
What Is Necessary For Diffusion To Occur
Mar 28, 2025
-
Color Of Flame Of Calcium Chloride
Mar 28, 2025
-
Differences Between Intermolecular And Intramolecular Forces
Mar 28, 2025
-
Identify The Functional Group In Each Compound
Mar 28, 2025
-
Political Results Of The Industrial Revolution
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Alkane Is The Isomer Of Butane Called 2 Methylpropane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
