Differences Between Intermolecular And Intramolecular Forces
Muz Play
Mar 28, 2025 · 7 min read
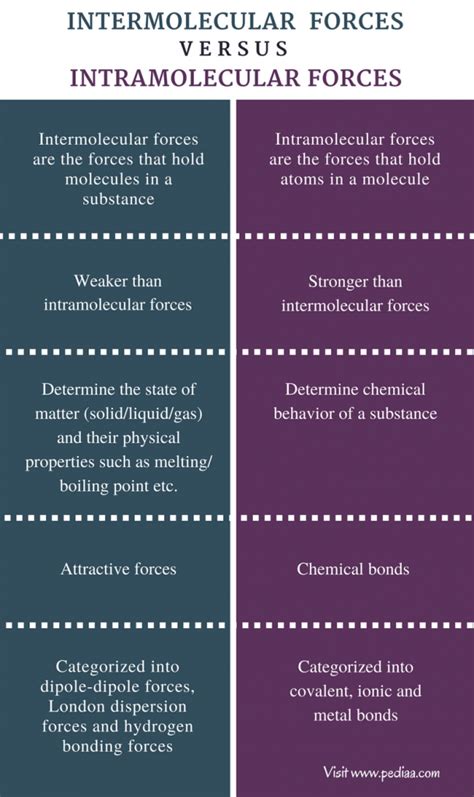
Table of Contents
Delving Deep into the Differences: Intermolecular vs. Intramolecular Forces
Understanding the fundamental forces that govern the behavior of molecules is crucial in chemistry. These forces, broadly categorized as intermolecular and intramolecular forces, dictate a wide range of properties, from boiling points and melting points to solubility and reactivity. While both types involve interactions between atoms, their nature and strength differ significantly. This article will provide a comprehensive exploration of these differences, clarifying the nuances and illustrating their impact on molecular systems.
What are Intermolecular Forces?
Intermolecular forces (IMFs) are the attractive or repulsive forces between separate molecules. These forces are significantly weaker than the intramolecular forces that hold atoms together within a molecule. The strength of IMFs plays a crucial role in determining the physical properties of substances, such as their state at room temperature (solid, liquid, or gas), boiling point, melting point, viscosity, and surface tension.
Types of Intermolecular Forces:
Several types of IMFs exist, each varying in strength and origin:
-
London Dispersion Forces (LDFs): These are the weakest type of IMF and are present in all molecules, regardless of polarity. They arise from temporary, instantaneous fluctuations in electron distribution around atoms, creating temporary dipoles. These temporary dipoles induce dipoles in neighboring molecules, leading to weak attractive forces. The strength of LDFs increases with the size and shape of the molecule, as larger molecules have more electrons, resulting in greater potential for temporary dipole formation.
-
Dipole-Dipole Forces: These forces occur between polar molecules, molecules with a permanent dipole moment due to unequal sharing of electrons in covalent bonds. The positive end of one polar molecule attracts the negative end of another, resulting in a stronger attraction than LDFs. The strength of dipole-dipole interactions is directly proportional to the magnitude of the dipole moment.
-
Hydrogen Bonding: This is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as fluorine, oxygen, or nitrogen) and is attracted to another electronegative atom in a nearby molecule. Hydrogen bonds are stronger than typical dipole-dipole interactions because of the high electronegativity difference and the small size of the hydrogen atom, allowing for close proximity between the atoms. They are responsible for many unique properties of water, such as its high boiling point and surface tension.
-
Ion-Dipole Forces: These forces are found in mixtures of ionic compounds and polar molecules. The positive or negative ion is attracted to the oppositely charged end of the polar molecule. These are generally stronger than dipole-dipole forces.
What are Intramolecular Forces?
Intramolecular forces are the attractive forces within a molecule. These forces are responsible for holding atoms together to form molecules. They are significantly stronger than intermolecular forces. The primary type of intramolecular force is the chemical bond.
Types of Intramolecular Forces (Chemical Bonds):
-
Covalent Bonds: These bonds involve the sharing of electrons between two atoms. Covalent bonds are formed between nonmetals and are characterized by relatively strong attractions. The strength of a covalent bond depends on the number of shared electron pairs (single, double, or triple bonds) and the electronegativity difference between the atoms. Stronger bonds result from a greater degree of electron sharing.
-
Ionic Bonds: These bonds involve the electrostatic attraction between oppositely charged ions. They are formed between metals and nonmetals, resulting from the transfer of electrons from a metal atom to a nonmetal atom. Ionic bonds are generally stronger than covalent bonds, and the strength is influenced by the charge and size of the ions. Larger charges and smaller ion sizes lead to stronger attractions.
-
Metallic Bonds: These bonds occur in metals and involve the delocalization of electrons among a lattice of metal atoms. The valence electrons are not associated with specific atoms but are free to move throughout the metal structure, creating a "sea" of electrons. This delocalization accounts for many properties of metals, such as high electrical and thermal conductivity and malleability.
Key Differences Between Intermolecular and Intramolecular Forces:
The table below summarizes the key distinctions between intermolecular and intramolecular forces:
| Feature | Intermolecular Forces | Intramolecular Forces |
|---|---|---|
| Nature | Attractive or repulsive forces between molecules | Attractive forces within a molecule |
| Strength | Weak | Strong |
| Type of Bond | Not a chemical bond; physical interactions | Chemical bond |
| Energy Involved | Lower energy changes (e.g., phase transitions) | Higher energy changes (e.g., bond breaking/formation) |
| Effect on Properties | Determine physical properties (boiling point, melting point, etc.) | Determine chemical properties and some aspects of physical properties (hardness, bond angles, etc.) |
| Examples | LDFs, dipole-dipole forces, hydrogen bonds, ion-dipole forces | Covalent bonds, ionic bonds, metallic bonds |
| Bond Breaking/Formation | No bond breaking/formation required for interaction | Bond breaking/formation required for interaction |
The Impact of Intermolecular and Intramolecular Forces on Properties:
The balance between intermolecular and intramolecular forces significantly influences the macroscopic properties of a substance.
Boiling Point and Melting Point: Stronger intermolecular forces lead to higher boiling and melting points because more energy is needed to overcome the attractive forces between molecules. For instance, water has a relatively high boiling point due to its strong hydrogen bonds.
Solubility: The solubility of a substance depends on the interactions between the solute and the solvent. Polar substances tend to dissolve in polar solvents due to dipole-dipole or hydrogen bond interactions. Nonpolar substances dissolve in nonpolar solvents because of LDFs. "Like dissolves like" is a useful principle to remember.
Viscosity: Viscosity is a measure of a liquid's resistance to flow. Liquids with strong intermolecular forces have higher viscosity because the molecules are more strongly attracted to each other and thus resist movement.
Surface Tension: Surface tension is the tendency of liquid surfaces to minimize their area. Liquids with strong intermolecular forces have higher surface tension because the molecules at the surface are strongly attracted to each other.
Examples Illustrating the Differences:
Let's consider some examples to highlight the differences between intermolecular and intramolecular forces:
Water (H₂O): Within each water molecule, strong covalent bonds hold the oxygen and hydrogen atoms together (intramolecular forces). However, the water molecules themselves are attracted to each other through hydrogen bonds (intermolecular forces). These hydrogen bonds are responsible for water's high boiling point, surface tension, and its ability to act as a solvent for many polar substances.
Diamond: In diamond, carbon atoms are strongly bonded together through covalent bonds (intramolecular forces) in a giant covalent lattice structure. The extremely strong intramolecular forces contribute to diamond's exceptional hardness and high melting point. The intermolecular forces in diamond are negligible compared to the strong covalent bonds.
Sodium Chloride (NaCl): Sodium chloride is an ionic compound held together by strong ionic bonds (intramolecular forces) between Na⁺ and Cl⁻ ions. The strength of these bonds results in a high melting point and hardness. The forces between individual NaCl crystals are relatively weak intermolecular forces (ionic interactions).
Conclusion:
Intermolecular and intramolecular forces are fundamentally different types of interactions that dictate the physical and chemical properties of matter. Intramolecular forces, specifically chemical bonds, are strong and hold atoms together within molecules. Intermolecular forces are weaker and act between separate molecules, influencing macroscopic properties such as boiling point, melting point, and solubility. Understanding the nature and strength of these forces is crucial for predicting and interpreting the behavior of molecules and materials. By considering both types of forces, we gain a more comprehensive understanding of the world around us at a molecular level. Further exploration into these concepts can lead to advancements in fields such as materials science, drug discovery, and nanotechnology, where manipulating molecular interactions is key to developing new technologies and applications.
Latest Posts
Latest Posts
-
What Are Two Divisions Of The Skeleton
Mar 31, 2025
-
When Is It Acceptable To Use A Personnel Platform
Mar 31, 2025
-
Packing Efficiency Of Body Centered Cubic
Mar 31, 2025
-
What Is Reversible And Irreversible Process In Thermodynamics
Mar 31, 2025
-
Moment Of Inertia Of Uniform Rod
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Differences Between Intermolecular And Intramolecular Forces . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
