What Is Necessary For Diffusion To Occur
Muz Play
Mar 28, 2025 · 6 min read
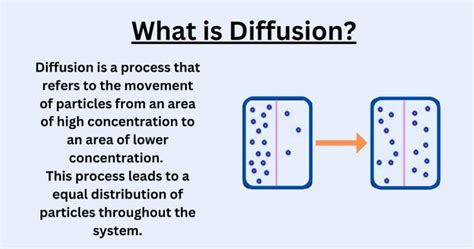
Table of Contents
- What Is Necessary For Diffusion To Occur
- Table of Contents
- What is Necessary for Diffusion to Occur?
- The Fundamental Requirements for Diffusion
- 1. A Concentration Gradient: The Driving Force
- 2. Random Molecular Motion: The Engine of Diffusion
- 3. A Medium for Movement: The Role of the Environment
- 4. Permeability of the Medium: Barriers and Pathways
- 5. Temperature: Speeding up the Process
- Factors Influencing the Rate of Diffusion
- Diffusion in Different Contexts
- Diffusion in Biology: A Fundamental Process of Life
- Diffusion in Chemistry: Reactions and Equilibria
- Diffusion in Materials Science: Controlling Material Properties
- Diffusion in Environmental Science: Pollution Dispersion and Remediation
- Conclusion: A Ubiquitous Process
- Latest Posts
- Latest Posts
- Related Post
What is Necessary for Diffusion to Occur?
Diffusion, a fundamental process in nature, is the net movement of anything (for example, atom, ions, molecules) from a region of higher concentration to a region of lower concentration. This movement continues until the concentration is uniform throughout. Understanding the conditions necessary for diffusion to occur is crucial in various fields, from biology and chemistry to materials science and environmental science. This article delves deep into the essential factors that drive this ubiquitous process.
The Fundamental Requirements for Diffusion
Several key factors are necessary for diffusion to take place effectively. These can be broadly categorized as:
1. A Concentration Gradient: The Driving Force
The most crucial requirement for diffusion is a concentration gradient. This simply means a difference in the concentration of the substance across a space. Molecules naturally move from an area of high concentration (where they are crowded) to an area of low concentration (where there's more space). This movement is driven by the inherent randomness of molecular motion—a concept we'll explore further. Without a concentration gradient, there's no driving force for diffusion, and the system remains at equilibrium. Imagine a drop of ink in a glass of water; the ink diffuses because its concentration is initially much higher within the drop compared to the surrounding water.
2. Random Molecular Motion: The Engine of Diffusion
Diffusion is a direct consequence of the constant, random motion of molecules. This motion, stemming from the kinetic energy possessed by all matter above absolute zero, is called Brownian motion. Molecules are in ceaseless chaotic movement, constantly colliding with each other and their surroundings. These collisions cause them to change direction and speed constantly. While individual molecular movements are random, the net movement of many molecules results in a directional flow from high to low concentration. The higher the temperature, the greater the kinetic energy, and consequently, the faster the rate of diffusion.
3. A Medium for Movement: The Role of the Environment
The substance undergoing diffusion needs a medium through which it can move. This medium can be a gas, liquid, or even a solid, although the rate of diffusion varies significantly between these states.
-
Gases: Gases have the highest diffusion rates due to the large distances between molecules and their high kinetic energy. Molecules in a gas can travel relatively long distances before colliding.
-
Liquids: Liquids have slower diffusion rates than gases because the molecules are closer together, leading to more frequent collisions. However, they still possess significant kinetic energy, allowing for substantial movement.
-
Solids: Diffusion in solids is the slowest because the molecules are tightly packed and their movement is significantly restricted. Diffusion in solids often occurs through interstitial spaces or vacancies within the crystal lattice structure.
The properties of the medium also affect diffusion. Factors like viscosity (resistance to flow) in liquids and density in both liquids and gases significantly impact the rate of diffusion. A more viscous liquid, for example, will hinder diffusion compared to a less viscous one.
4. Permeability of the Medium: Barriers and Pathways
The permeability of the medium through which diffusion occurs is another critical factor. A permeable medium allows molecules to pass through easily, while an impermeable medium prevents or significantly restricts movement. Cell membranes, for example, are selectively permeable, allowing certain molecules to pass through while preventing others. The presence of pores, channels, or other pathways in the medium can significantly enhance diffusion rates.
5. Temperature: Speeding up the Process
As mentioned earlier, temperature plays a pivotal role in diffusion. Higher temperatures increase the kinetic energy of molecules, leading to faster movement and a higher rate of diffusion. This is because increased kinetic energy translates to more frequent and forceful collisions, propelling molecules across the concentration gradient more rapidly. Conversely, lower temperatures slow down diffusion.
Factors Influencing the Rate of Diffusion
While the factors above are essential for diffusion to occur at all, several other factors influence how quickly diffusion takes place. These include:
-
Mass of the diffusing substance: Heavier molecules diffuse more slowly than lighter molecules because they possess less kinetic energy at the same temperature.
-
Surface area: A larger surface area facilitates faster diffusion because more molecules can simultaneously cross the boundary between regions of differing concentrations.
-
Distance: The rate of diffusion decreases with increasing distance. The further molecules have to travel, the longer it takes for the concentration gradient to even out.
-
Pressure (in gases): Higher pressure in gases increases the frequency of collisions, leading to a faster diffusion rate.
Diffusion in Different Contexts
Understanding the necessary conditions for diffusion is vital in a wide array of scientific fields. Let's look at a few examples:
Diffusion in Biology: A Fundamental Process of Life
Diffusion is fundamental to numerous biological processes. For instance:
-
Gas exchange in the lungs: Oxygen diffuses from the alveoli (air sacs in the lungs) into the blood, while carbon dioxide diffuses from the blood into the alveoli. This relies on a concentration gradient and the permeability of the alveolar membranes.
-
Nutrient absorption in the intestines: Nutrients from digested food diffuse across the intestinal lining into the bloodstream.
-
Neurotransmission: Neurotransmitters diffuse across the synaptic cleft (the gap between nerve cells) to transmit signals.
-
Cellular respiration: The diffusion of oxygen and glucose into cells and the diffusion of carbon dioxide out of cells are essential for cellular energy production.
Diffusion in Chemistry: Reactions and Equilibria
Diffusion plays a crucial role in chemical reactions, particularly those involving gases or liquids. The rate of a reaction often depends on how quickly reactants can diffuse towards each other. Diffusion also helps establish chemical equilibria, where the rates of forward and reverse reactions are equal.
Diffusion in Materials Science: Controlling Material Properties
Diffusion is used in materials science to modify the properties of materials. Processes like doping semiconductors or heat treating metals rely on the diffusion of atoms into the material to alter its electrical conductivity, strength, or other properties.
Diffusion in Environmental Science: Pollution Dispersion and Remediation
Diffusion is crucial in understanding how pollutants are dispersed in the atmosphere and water. The rate of diffusion of pollutants influences the extent of contamination and the effectiveness of remediation strategies.
Conclusion: A Ubiquitous Process
Diffusion is a ubiquitous and essential process that governs many phenomena across various scientific disciplines. Understanding the conditions necessary for its occurrence—a concentration gradient, random molecular motion, a medium for movement, medium permeability, and the influence of temperature—is key to comprehending these phenomena and harnessing the process for technological advancements. By controlling the factors that influence diffusion, we can design materials with specific properties, manage environmental pollution effectively, and gain deeper insights into biological processes. The seemingly simple movement of molecules from high to low concentration is, in reality, a complex and crucial process that shapes our world.
Latest Posts
Latest Posts
-
Four Ways To Represent A Function
Mar 31, 2025
-
What Is The Functional Unit Of Heredity
Mar 31, 2025
-
Cracking The Code Of Life Answer Key
Mar 31, 2025
-
Delta H Is Negative Exothermic Or Endothermic
Mar 31, 2025
-
Raising And Lowering Operators Angular Momentum
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is Necessary For Diffusion To Occur . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
