Understanding Theoretical Actual And Percent Yield
Muz Play
Mar 25, 2025 · 5 min read
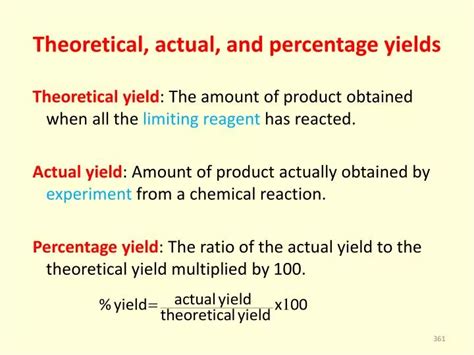
Table of Contents
Understanding Theoretical, Actual, and Percent Yield: A Comprehensive Guide
Chemical reactions are the cornerstone of chemistry, enabling the transformation of matter from one form to another. Understanding the efficiency of these transformations is crucial, and that's where the concepts of theoretical, actual, and percent yield come into play. These three terms are fundamental to stoichiometry, allowing chemists to assess the success of a reaction and identify areas for improvement. This comprehensive guide will delve into each concept, explain their calculations, and provide examples to solidify your understanding.
Theoretical Yield: The Ideal Outcome
The theoretical yield represents the maximum amount of product that can be formed from a given amount of reactant, assuming the reaction proceeds completely and without any loss or side reactions. It's the ideal scenario, the perfect world of chemistry where every molecule reacts as expected. To calculate the theoretical yield, we rely on stoichiometry – the relationship between the amounts of reactants and products in a balanced chemical equation.
Steps to Calculate Theoretical Yield:
-
Balance the chemical equation: This ensures the correct mole ratios between reactants and products are established.
-
Convert reactant mass to moles: Use the molar mass of the limiting reactant (the reactant that gets completely consumed first) to convert its mass (in grams) to moles.
-
Use mole ratios from the balanced equation: Determine the mole ratio between the limiting reactant and the desired product. Multiply the moles of the limiting reactant by this ratio to find the moles of the product formed.
-
Convert moles of product to mass: Use the molar mass of the product to convert the moles of product to grams, giving you the theoretical yield.
Example:
Consider the reaction: 2H₂ + O₂ → 2H₂O
Let's say we react 4 grams of hydrogen (H₂) with excess oxygen (O₂).
-
Balanced equation: The equation is already balanced.
-
Moles of H₂: Molar mass of H₂ = 2 g/mol. Moles of H₂ = 4 g / 2 g/mol = 2 moles.
-
Mole ratio: From the balanced equation, the mole ratio of H₂ to H₂O is 2:2, or 1:1. Therefore, 2 moles of H₂ will produce 2 moles of H₂O.
-
Mass of H₂O: Molar mass of H₂O = 18 g/mol. Mass of H₂O = 2 moles * 18 g/mol = 36 grams.
Therefore, the theoretical yield of water is 36 grams. This assumes perfect reaction conditions and complete conversion of hydrogen to water.
Actual Yield: The Reality of the Reaction
The actual yield is the amount of product that is actually obtained from a chemical reaction in a laboratory setting. This is the real-world outcome, taking into account all the imperfections and limitations of the experimental procedure. Factors that can affect the actual yield include:
- Incomplete reactions: Not all reactants might react, leaving some unreacted.
- Side reactions: Unwanted reactions can occur, consuming reactants and producing unwanted byproducts.
- Loss of product during purification: Some product might be lost during the separation and purification steps.
- Experimental errors: Inaccurate measurements, improper techniques, or equipment malfunction can all reduce the actual yield.
The actual yield is always determined experimentally and is usually less than the theoretical yield. It's measured by isolating and weighing the purified product after the reaction is complete.
Percent Yield: A Measure of Efficiency
The percent yield is a crucial measure of the efficiency of a chemical reaction. It expresses the ratio of the actual yield to the theoretical yield, multiplied by 100%. A higher percent yield indicates a more efficient reaction.
Formula for Percent Yield:
Percent Yield = (Actual Yield / Theoretical Yield) x 100%
Example:
Using the previous example, let's assume that in the experiment, only 30 grams of water were actually obtained.
Actual Yield = 30 grams
Theoretical Yield = 36 grams
Percent Yield = (30 grams / 36 grams) x 100% = 83.33%
This means the reaction was 83.33% efficient. The remaining 16.67% represents losses due to incomplete reaction, side reactions, or experimental errors.
Factors Affecting Percent Yield
Several factors can significantly influence the percent yield of a chemical reaction. Understanding these factors allows chemists to optimize reaction conditions to maximize the yield.
Limiting Reactant:
The reactant present in the smallest stoichiometric amount determines the maximum amount of product that can be formed. If one reactant is in excess, the limiting reactant dictates the theoretical and actual yields.
Reaction Conditions:
Temperature, pressure, concentration, and the presence of catalysts can greatly influence reaction rates and yields. Optimizing these conditions is crucial for achieving higher yields.
Side Reactions:
Unwanted side reactions can consume reactants and reduce the amount of desired product formed. Careful control of reaction conditions can minimize side reactions.
Purification Techniques:
Losses during the purification of the product are common. Efficient and effective purification methods are essential to minimize these losses and improve the actual yield.
Experimental Errors:
Errors in measurement, technique, or equipment can lead to lower actual yields. Careful attention to detail and the use of precise equipment are essential for minimizing errors.
Importance of Understanding Yield
Understanding theoretical, actual, and percent yields is crucial for several reasons:
- Assessing reaction efficiency: Percent yield provides a direct measure of how well a reaction performs.
- Optimizing reaction conditions: By analyzing the yield, chemists can identify areas for improvement in the reaction procedure.
- Predicting the amount of product: Theoretical yield helps predict the amount of product that can be expected from a given amount of reactant.
- Industrial applications: In industrial settings, maximizing yield is crucial for economic reasons. Higher yields translate to lower costs and greater profits.
- Research and development: Understanding yields is critical in the development of new chemical processes and materials.
Conclusion
Theoretical, actual, and percent yields are fundamental concepts in chemistry that allow for a quantitative assessment of the efficiency of chemical reactions. By understanding these concepts and the factors that influence them, chemists can optimize reaction conditions, minimize losses, and achieve higher yields, leading to more efficient and cost-effective chemical processes. Mastering these calculations is essential for success in any chemical endeavor, whether it's in a laboratory setting, an industrial plant, or a research environment. The careful consideration of each stage, from balancing the equation to purifying the product, is vital in maximizing the yield and understanding the true efficiency of the chemical transformation.
Latest Posts
Latest Posts
-
Electric Field Of A Disk Of Charge
Mar 28, 2025
-
Active Transport Must Function Continuously Because
Mar 28, 2025
-
The Basic Functional Unit Of The Kidney Is
Mar 28, 2025
-
Weak Development Of Support Examples In Essay
Mar 28, 2025
-
On The Weak Acid Strong Base Titration Curve
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Understanding Theoretical Actual And Percent Yield . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
