How To Hydrolyze Activated Carboxylic Acid Ester
Muz Play
Mar 28, 2025 · 5 min read
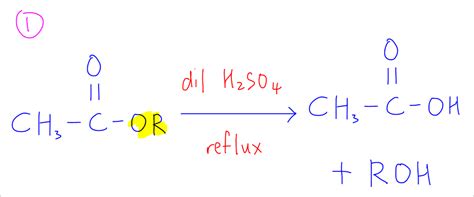
Table of Contents
How to Hydrolyze Activated Carboxylic Acid Esters: A Comprehensive Guide
Hydrolysis of carboxylic acid esters is a fundamental reaction in organic chemistry with wide-ranging applications in various fields, from the synthesis of pharmaceuticals and polymers to the breakdown of fats and oils in biological systems. While simple esters can be hydrolyzed under relatively harsh conditions (e.g., strong acid or base), activated carboxylic acid esters, which possess electron-withdrawing groups near the ester carbonyl, are significantly more reactive and can be hydrolyzed under milder conditions. This enhanced reactivity makes them crucial building blocks in numerous synthetic strategies. This article delves into the mechanisms and methods involved in hydrolyzing activated carboxylic acid esters.
Understanding Activated Carboxylic Acid Esters
The key to understanding the enhanced reactivity of activated esters lies in the electronic effects of substituents adjacent to the carbonyl group. Electron-withdrawing groups (EWGs), such as nitro (-NO₂), cyano (-CN), or halogen atoms (e.g., -Cl, -Br), increase the electrophilicity of the carbonyl carbon. This increased electrophilicity makes the carbonyl carbon more susceptible to nucleophilic attack, the crucial first step in the hydrolysis reaction.
Common examples of activated esters include:
- p-Nitrophenyl esters: The strong electron-withdrawing nitro group significantly enhances reactivity.
- Pentafluorophenyl esters: The multiple fluorine atoms create a highly electron-deficient aromatic ring, further activating the ester.
- N-Hydroxysuccinimide (NHS) esters: The cyclic imide structure stabilizes the leaving group, making hydrolysis favorable.
- Thioesters: The sulfur atom, being less electronegative than oxygen, makes the carbonyl carbon more electrophilic compared to oxygen esters.
Mechanisms of Hydrolysis
The hydrolysis of activated carboxylic acid esters generally proceeds through one of two main mechanisms:
1. Acid-Catalyzed Hydrolysis
This mechanism involves protonation of the carbonyl oxygen, followed by nucleophilic attack by water. The protonation increases the electrophilicity of the carbonyl carbon, making it more susceptible to attack by water. The subsequent steps involve proton transfers and the eventual expulsion of the activated leaving group, resulting in the formation of a carboxylic acid.
Step-by-step mechanism:
- Protonation: A proton from the acid catalyst protonates the carbonyl oxygen, increasing the electrophilicity of the carbonyl carbon.
- Nucleophilic attack: A water molecule acts as a nucleophile, attacking the electrophilic carbonyl carbon.
- Tetrahedral intermediate formation: A tetrahedral intermediate is formed.
- Proton transfer: A proton is transferred from the hydroxyl group to one of the oxygen atoms.
- Elimination: The activated leaving group departs, regenerating the carbonyl group.
- Deprotonation: A proton is removed from the hydroxyl group, yielding the carboxylic acid.
2. Base-Catalyzed Hydrolysis (Saponification)
Base-catalyzed hydrolysis, also known as saponification, involves the attack of a hydroxide ion (OH⁻) on the carbonyl carbon. The hydroxide ion is a strong nucleophile, and the electron-withdrawing groups on the activated ester enhance the electrophilicity of the carbonyl carbon, making the reaction faster than with non-activated esters. The reaction proceeds through a similar tetrahedral intermediate, but the leaving group departs as an alkoxide ion, which is subsequently protonated to form the corresponding alcohol.
Step-by-step mechanism:
- Nucleophilic attack: The hydroxide ion attacks the carbonyl carbon.
- Tetrahedral intermediate formation: A tetrahedral intermediate is formed.
- Elimination: The activated leaving group departs as an alkoxide ion.
- Protonation: The alkoxide ion is protonated to form the corresponding alcohol.
Factors Affecting Hydrolysis Rate
Several factors influence the rate of hydrolysis of activated carboxylic acid esters:
- Nature of the activating group: Stronger electron-withdrawing groups lead to faster hydrolysis.
- Solvent: Polar protic solvents (like water or alcohols) generally favor hydrolysis.
- Temperature: Higher temperatures increase the reaction rate.
- pH: Acidic or basic conditions can significantly affect the reaction rate, with the optimal pH depending on the specific ester.
- Steric hindrance: Bulky groups around the ester can hinder nucleophilic attack, slowing down the reaction.
Methods for Hydrolyzing Activated Carboxylic Acid Esters
The choice of method for hydrolyzing activated esters depends on the specific ester and desired reaction conditions. Here are some common approaches:
1. Aqueous Acid Hydrolysis
This method is suitable for many activated esters and involves treating the ester with a dilute aqueous acid solution (e.g., HCl or H₂SO₄) at elevated temperatures. The acid acts as a catalyst, accelerating the reaction.
2. Aqueous Base Hydrolysis (Saponification)
This method utilizes an aqueous solution of a strong base (e.g., NaOH or KOH) to hydrolyze the ester. It is particularly effective for esters with relatively stable leaving groups. The resulting carboxylate salt can be easily acidified to obtain the free carboxylic acid.
3. Enzymatic Hydrolysis
Certain enzymes, such as esterases and lipases, can catalyze the hydrolysis of esters under mild conditions. This method is highly specific and environmentally friendly, making it attractive for certain applications.
4. Microwave-Assisted Hydrolysis
Microwave irradiation can accelerate the hydrolysis process by providing rapid and efficient heating. This technique can significantly reduce reaction times and improve yields.
5. Ultrasound-Assisted Hydrolysis
Similar to microwave-assisted hydrolysis, ultrasound can enhance the reaction rate by creating cavitation bubbles that promote mass transfer and increase the surface area available for reaction.
Applications of Hydrolyzed Activated Carboxylic Acid Esters
The products of hydrolyzing activated carboxylic acid esters – carboxylic acids and alcohols – are valuable building blocks in organic synthesis. These compounds are used in the preparation of a vast array of molecules, including:
- Pharmaceuticals: Many pharmaceuticals contain carboxylic acid or alcohol functionalities derived from hydrolyzed esters.
- Polymers: Hydrolyzed esters serve as monomers in the synthesis of various polymers.
- Biofuels: The hydrolysis of fatty acid esters (triglycerides) is a crucial step in biodiesel production.
- Surfactants: Hydrolyzed esters can be used to synthesize surfactants with desirable properties.
- Flavors and fragrances: Many esters are hydrolyzed to produce carboxylic acids and alcohols used in flavor and fragrance formulations.
Conclusion
Hydrolyzing activated carboxylic acid esters is a powerful and versatile technique with broad applications in organic chemistry and related fields. Understanding the mechanisms, influencing factors, and available methods allows chemists to tailor the reaction conditions to achieve optimal yields and selectivity. The resulting carboxylic acids and alcohols are essential building blocks for the synthesis of a wide range of valuable compounds. The choice of hydrolysis method depends heavily on the specific ester, desired reaction conditions, and the scale of the operation. Careful consideration of these factors is crucial for successful execution of the reaction. Further research continues to explore novel and improved methods for hydrolyzing activated esters, promising even greater efficiency and sustainability in the future.
Latest Posts
Latest Posts
-
Which One Is Good Insulator Metals Metalloids Or Nonmetals
Mar 31, 2025
-
Why Are Covalent Compounds Not Conductive
Mar 31, 2025
-
What Does It Mean To Be An Artist
Mar 31, 2025
-
Reaction Of Grignard Reagent With Water
Mar 31, 2025
-
What Type Of Population Density Dependence Focuses On Abiotic Factors
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How To Hydrolyze Activated Carboxylic Acid Ester . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
