How To Identify Molecular Ion Peak
Muz Play
Mar 18, 2025 · 7 min read
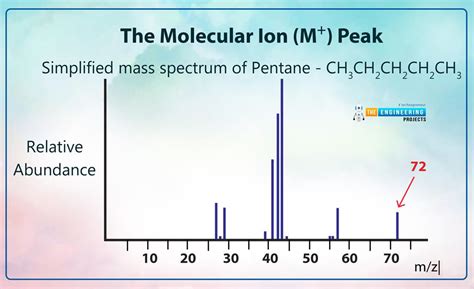
Table of Contents
How to Identify the Molecular Ion Peak in Mass Spectrometry
Mass spectrometry (MS) is a powerful analytical technique used to determine the mass-to-charge ratio (m/z) of ions. One of the most crucial aspects of interpreting a mass spectrum is identifying the molecular ion peak, often denoted as M+. This peak represents the mass of the intact molecule, providing fundamental information about the molecule's molecular weight and elemental composition. However, identifying the molecular ion peak isn't always straightforward, and requires a careful understanding of several factors. This article will comprehensively guide you through the process, covering various techniques and considerations.
Understanding the Molecular Ion Peak (M+)
The molecular ion peak (M+) is formed when a molecule in the ionization source loses one electron, creating a radical cation. This process is crucial because it provides the molecular weight of the compound. The m/z value of this peak directly corresponds to the molecular weight of the molecule.
Why is Identifying M+ Crucial?
Identifying the M+ peak is the cornerstone of mass spectrometry data analysis. It allows you to:
- Determine the molecular weight: This is the most fundamental piece of information obtained from a mass spectrum.
- Deduce the empirical formula: Combined with other spectral data (like isotopic patterns), you can determine the elemental composition.
- Identify the compound: By comparing the molecular weight and fragmentation pattern with known compounds in databases, you can potentially identify the unknown molecule.
- Formulate a structure: The fragmentation pattern, in conjunction with the molecular ion peak, helps determine the structure of the compound.
Factors Affecting the Molecular Ion Peak's Intensity and Visibility
The intensity of the molecular ion peak can vary significantly depending on several factors:
1. Molecular Structure:
- Stability of the radical cation: Molecules that form relatively stable radical cations tend to exhibit more intense M+ peaks. This stability depends on factors like resonance stabilization, electron delocalization, and the presence of electron-donating or withdrawing groups. Aromatic compounds, for example, often show more intense M+ peaks.
- Fragmentation pathways: Molecules with readily available fragmentation pathways may produce weak or absent M+ peaks because the molecule prefers to fragment into smaller, more stable ions.
- Molecular size and complexity: Larger, more complex molecules may undergo extensive fragmentation, leading to a less prominent or even absent M+ peak.
2. Ionization Technique:
Different ionization techniques produce varying degrees of fragmentation.
- Electron Ionization (EI): EI is a hard ionization technique that often causes extensive fragmentation, resulting in a less intense or even absent M+ peak, particularly for larger molecules. However, EI provides highly reproducible fragmentation patterns useful for compound identification.
- Chemical Ionization (CI): CI is a softer ionization technique that produces less fragmentation, resulting in a more prominent M+ peak, particularly for larger molecules. It's particularly useful for determining the molecular weight when EI fails to give a strong M+.
- Electrospray Ionization (ESI): ESI is a very soft ionization technique commonly used in liquid chromatography-mass spectrometry (LC-MS), often resulting in abundant M+ peaks, even for large and complex molecules. It minimizes fragmentation, making it ideal for analyzing biomolecules.
- Matrix-Assisted Laser Desorption/Ionization (MALDI): MALDI is another soft ionization technique often used for analyzing large biomolecules like proteins and polymers. It typically produces abundant M+ peaks (or [M+H]+ peaks).
3. Instrument Parameters:
Instrument parameters, like the voltage in the ionization source and the acceleration voltage, significantly impact fragmentation. Higher voltages generally lead to more fragmentation, resulting in a weaker M+ peak.
Identifying the Molecular Ion Peak: Practical Strategies
Identifying the molecular ion peak requires a systematic approach combining visual inspection and logical reasoning.
1. Visual Inspection:
- Look for the highest m/z value: While not always the case (due to potential adducts or other ions), the molecular ion peak often has the highest m/z value in the spectrum, especially with softer ionization techniques.
- Consider the isotopic pattern: Many elements have isotopes, creating characteristic isotopic patterns. The presence of a peak with the expected isotopic ratio is a strong indication of the molecular ion peak. Chlorine (35Cl and 37Cl) and Bromine (79Br and 81Br) are particularly useful for this.
- Examine the fragmentation pattern: Fragment ions are often observed at lower m/z values than the molecular ion. Look for peaks that appear to be fragments of a larger ion, which might provide clues about the molecular weight.
2. Using Isotopic Ratios for Confirmation:
The presence of isotopes allows you to confirm the molecular ion peak. For instance:
- Chlorine: A compound containing one chlorine atom will show two peaks with a 3:1 intensity ratio, separated by 2 m/z units.
- Bromine: A compound containing one bromine atom will show two peaks with a 1:1 intensity ratio, separated by 2 m/z units.
- Other elements: Other elements like carbon (¹²C and ¹³C) also exhibit isotopic patterns, although they are less pronounced. These can be used to confirm the presence of multiple atoms of these elements. Software often performs this isotopic pattern analysis automatically.
3. Utilizing Nitrogen Rule:
The nitrogen rule is a helpful guideline in organic chemistry. It states that if a molecule contains an odd number of nitrogen atoms, its molecular weight will be odd. Conversely, if it contains an even number of nitrogen atoms (or no nitrogen), its molecular weight will be even. This rule can aid in determining if a potential M+ peak is plausible.
4. Analyzing Fragmentation Patterns:
Analyzing fragment ions can help identify the molecular ion peak indirectly. For example:
- Look for recurring mass differences: Identify consistent mass differences between peaks. This can be indicative of specific fragment losses.
- Consider common fragmentation pathways: Knowing typical fragmentation pathways for different functional groups can help predict fragment ion masses and potentially identify the molecular ion. For instance, the loss of a methyl group (15 Da) or a water molecule (18 Da) are common.
5. Employing Mass Spectrometry Software:
Modern mass spectrometry software significantly simplifies the identification process. Features like:
- Automatic peak detection and annotation: Many software packages automatically detect and annotate peaks.
- Isotopic pattern analysis: This function automatically analyzes isotopic patterns to confirm potential molecular ion peaks.
- Database searching: Searching libraries of known compounds based on the molecular weight and fragmentation pattern can help identify the unknown compound.
Challenges in Identifying the Molecular Ion Peak
Despite the strategies mentioned above, several challenges can still hinder the accurate identification of the molecular ion peak:
- Weak or absent M+ peak: In some cases, especially with hard ionization techniques and large or unstable molecules, the M+ peak may be weak or entirely absent, making identification difficult.
- Adduct ion formation: Ions can form adducts with solvent molecules or other species in the ionization source, resulting in peaks with masses higher than the true molecular weight.
- Multiple charge states: In ESI and MALDI, molecules can carry multiple charges, leading to multiple peaks for the same molecule at different m/z values. Identifying the true molecular weight requires considering these charge states.
- Complex spectra: In mixtures, multiple compounds contribute to the spectrum, making it challenging to isolate the molecular ion peak of each individual component.
- Uncertainties in assignment: Even with careful analysis, there can still be uncertainties in assigning the molecular ion peak, requiring additional techniques for confirmation.
Conclusion
Identifying the molecular ion peak is a critical step in mass spectrometry data analysis. It provides invaluable information about the molecule's molecular weight and can be used to deduce its elemental composition, structure, and ultimately, its identity. Successfully identifying the M+ peak requires a deep understanding of the factors influencing its intensity, coupled with the application of appropriate strategies, including careful visual inspection, isotopic pattern analysis, fragmentation pattern analysis, the use of software, and awareness of potential challenges. The combination of these techniques will enhance your ability to extract maximum information from your mass spectral data. Remember to always approach mass spectrum interpretation systematically and critically evaluate potential candidates for the molecular ion peak.
Latest Posts
Latest Posts
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
-
Difference Between A Strong And Weak Acid
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How To Identify Molecular Ion Peak . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
