How To Interpret Minimum Inhibitory Concentration
Muz Play
Mar 19, 2025 · 6 min read
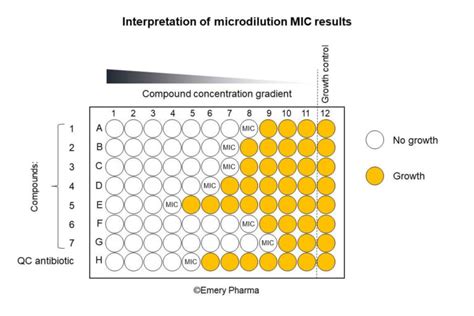
Table of Contents
How to Interpret Minimum Inhibitory Concentration (MIC) Results: A Comprehensive Guide
Minimum Inhibitory Concentration (MIC) is a crucial parameter in microbiology and antimicrobial susceptibility testing. It represents the lowest concentration of an antimicrobial drug that will inhibit the visible growth of a microorganism. Understanding how to interpret MIC results is vital for clinicians, researchers, and anyone involved in infectious disease management. This comprehensive guide will delve into the intricacies of MIC interpretation, covering its significance, methodologies, reporting, and limitations.
Understanding the Fundamentals of MIC
Before diving into interpretation, let's solidify our understanding of the core concept. The MIC is determined through various laboratory methods, most commonly broth microdilution or agar dilution. In essence, the test involves exposing a standardized inoculum of bacteria (or other microorganisms) to a range of antimicrobial concentrations. The lowest concentration preventing visible bacterial growth after incubation is defined as the MIC.
The Significance of MIC Values
MIC values are not simply numbers; they hold significant clinical implications. They provide a crucial piece of information for guiding treatment decisions:
-
Treatment Selection: MIC helps clinicians select the most appropriate antimicrobial agent for a specific infection. A lower MIC generally indicates a greater likelihood of successful treatment.
-
Dosage Adjustments: MIC data can inform decisions regarding appropriate drug dosage. Higher MIC values might necessitate higher doses or more prolonged therapy to achieve effective antimicrobial concentrations at the infection site.
-
Monitoring Antimicrobial Resistance: Tracking MIC values over time allows for monitoring the emergence and spread of antimicrobial resistance within a bacterial population. Increasing MIC values for a particular drug indicate the development of resistance.
-
Research and Development: MIC data is essential in the development and evaluation of new antimicrobial agents. It helps researchers assess the potency and spectrum of activity of novel drugs.
Methods for Determining MIC
Several methods exist for determining the MIC, each with its strengths and limitations. The most commonly used include:
1. Broth Microdilution
This is the gold standard method, providing high precision and reproducibility. Serial dilutions of the antimicrobial agent are prepared in a broth medium, and a standardized bacterial inoculum is added to each well. After incubation, the lowest concentration preventing visible growth is recorded as the MIC.
2. Agar Dilution
Similar to broth microdilution, this method involves preparing a series of agar plates containing different antimicrobial concentrations. A standardized inoculum is spread onto each plate, and the lowest concentration inhibiting growth is determined after incubation. While less precise than broth microdilution, agar dilution is simpler and less expensive.
3. Gradient Strip Methods (Etest)
This commercial method uses pre-formed strips containing a gradient of antimicrobial concentrations. The strip is placed on an inoculated agar plate, and the MIC is determined by reading the point of intersection between the growth inhibition ellipse and the strip. Etest offers a convenient and cost-effective alternative to broth and agar dilution, but its accuracy can be affected by factors such as bacterial inoculum size and growth conditions.
Interpreting MIC Results: A Practical Guide
Interpreting MIC results involves comparing the obtained value to established breakpoints. These breakpoints are predefined MIC values that categorize bacterial isolates as susceptible, intermediate, or resistant to a particular antimicrobial agent. These breakpoints are established by organizations like the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST). These organizations periodically update their breakpoints based on ongoing research and evolving epidemiological data.
Understanding Susceptible, Intermediate, and Resistant Categories:
-
Susceptible (S): The bacterial isolate is inhibited by the usually achievable concentrations of the antimicrobial agent in the patient's body. Treatment with the drug is expected to be successful.
-
Intermediate (I): The bacterial isolate's growth is inhibited by higher concentrations of the antimicrobial agent than usually achievable in the patient's body. Treatment with the drug may be successful, but only under specific circumstances (higher doses, targeted delivery). Further testing or alternative treatments might be considered.
-
Resistant (R): The bacterial isolate is not inhibited by usually achievable concentrations of the antimicrobial agent. Treatment with the drug is unlikely to be successful, and alternative treatment strategies are needed.
Factors Influencing MIC Interpretation:
Several factors beyond the raw MIC value can influence interpretation and treatment decisions:
-
Pharmacokinetic and Pharmacodynamic Properties: Understanding the drug's concentration in the body (pharmacokinetics) and its relationship to bacterial killing (pharmacodynamics) is crucial. Some drugs achieve high concentrations at the infection site, even if the MIC is relatively high.
-
Site of Infection: The location of the infection influences the achievable drug concentration. For example, achieving therapeutic concentrations in the brain or bone might be more challenging compared to achieving concentrations in the bloodstream.
-
Host Factors: Patient-specific factors such as age, immune status, and kidney or liver function can affect the drug's efficacy.
-
Combination Therapy: Using multiple antimicrobial agents simultaneously might enhance the overall effect, even if the MIC for individual drugs is relatively high.
Beyond MIC: Additional Considerations
While MIC is a key parameter, it's essential to consider other factors for comprehensive assessment:
-
Minimum Bactericidal Concentration (MBC): This indicates the lowest concentration of an antimicrobial agent that kills a certain percentage of bacteria (typically 99.9%). MBC provides a more robust measure of antimicrobial activity than MIC alone, particularly for organisms requiring bactericidal therapy.
-
Time-Kill Curves: These curves assess the killing kinetics of an antimicrobial agent over time. They provide a more detailed understanding of the antimicrobial's activity than MIC or MBC alone, helping to determine the best dosage regimen.
-
Post-Antibiotic Effect (PAE): This refers to the persistent suppression of bacterial growth even after the antimicrobial agent is removed. A longer PAE can contribute to improved treatment outcomes.
-
Clinical Outcomes: Ultimately, the effectiveness of antimicrobial therapy is judged by clinical improvement in the patient. MIC should be considered alongside clinical assessment, other lab results and the patient's response to treatment.
Limitations of MIC Testing
It's important to acknowledge the limitations of MIC testing:
-
In vitro versus in vivo: MIC testing is performed in a laboratory setting and doesn't perfectly reflect the complex environment within a patient's body.
-
Standardization challenges: Slight variations in testing methods and conditions can impact MIC results.
-
Lack of information on synergy or antagonism: MIC tests typically assess single antimicrobial agents, and don't provide information on how different drugs interact.
Practical Applications and Future Directions
The interpretation of MIC results is a cornerstone of antimicrobial stewardship. Clinicians use this data to make informed decisions about choosing the most effective antimicrobial agents, optimizing dosing strategies, and monitoring the emergence of resistance. With the rising threat of antimicrobial resistance, accurate and timely MIC testing is vital.
Future directions in MIC interpretation include:
-
Development of more sophisticated models: Integrating pharmacokinetic and pharmacodynamic parameters into MIC interpretation could improve the prediction of clinical outcomes.
-
Improved standardization of methods: This would enhance the consistency and reliability of MIC results across different laboratories.
-
Exploration of alternative testing methods: Rapid diagnostic techniques could accelerate the process of identifying the MIC, facilitating timely treatment decisions.
-
Integrating genomic data: Genomic information could provide insights into the mechanisms of resistance, further refining MIC interpretation and guiding treatment strategies.
In conclusion, understanding how to interpret MIC results is crucial for effective infection management. By considering the MIC in conjunction with other clinical and laboratory data, clinicians can optimize treatment strategies, improve patient outcomes, and combat the growing threat of antimicrobial resistance. This comprehensive guide provides a framework for understanding and interpreting MIC data, acknowledging both its significance and limitations. Remember to always consult with relevant guidelines and experts for specific interpretations and treatment recommendations.
Latest Posts
Latest Posts
-
What Is A Ligand In Biology
Mar 19, 2025
-
Can A Removable Discontinuity Be A Local Maximum
Mar 19, 2025
-
Movement Of Energy During Phase Transitions
Mar 19, 2025
-
Where Are The Reactants Located In A Chemical Equation
Mar 19, 2025
-
Does Fluorine Cause 13c Nmr Splitting
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How To Interpret Minimum Inhibitory Concentration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
