How To Read Ir Spectra Graphs
Muz Play
Mar 24, 2025 · 6 min read
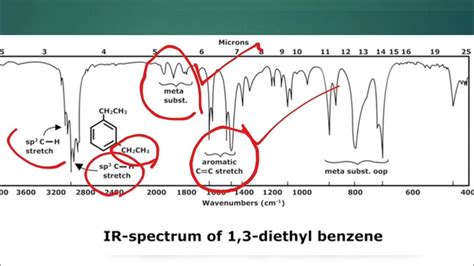
Table of Contents
How to Read IR Spectra Graphs: A Comprehensive Guide
Infrared (IR) spectroscopy is a powerful analytical technique used to identify functional groups within a molecule. Understanding how to interpret IR spectra is crucial for chemists, material scientists, and anyone working with molecular characterization. This comprehensive guide will walk you through the basics of IR spectroscopy, explain the key features of an IR spectrum, and provide practical tips for interpreting these graphs effectively.
Understanding the Fundamentals of Infrared Spectroscopy
Infrared spectroscopy relies on the principle of molecular vibrations. Molecules are not static entities; their atoms are constantly vibrating at various frequencies. When infrared radiation interacts with a molecule, it can be absorbed if the frequency of the radiation matches the frequency of a specific vibrational mode within the molecule. This absorption is what we observe as peaks in an IR spectrum.
Types of Molecular Vibrations
Molecules can exhibit several types of vibrations, including:
- Stretching vibrations: These involve changes in the bond length between two atoms. Stretching can be symmetric or asymmetric, depending on the movement of the atoms involved.
- Bending vibrations: These involve changes in the bond angle between two or more atoms. Bending vibrations can be further categorized into scissoring, rocking, wagging, and twisting.
The specific frequencies at which these vibrations occur depend on several factors, including:
- The mass of the atoms involved: Heavier atoms vibrate at lower frequencies.
- The bond strength: Stronger bonds vibrate at higher frequencies.
- The surrounding chemical environment: The electronic interactions with neighboring atoms can influence vibrational frequencies.
Deciphering the IR Spectrum: A Step-by-Step Approach
An IR spectrum is a plot of infrared absorption intensity (usually in transmittance or absorbance units) versus wavenumber (cm⁻¹). The wavenumber is inversely proportional to wavelength and is a more convenient unit for expressing IR data. Higher wavenumbers correspond to higher frequencies and stronger bonds.
Here's a step-by-step approach to reading and interpreting an IR spectrum:
1. Identify the Functional Group Region (4000-1500 cm⁻¹):
This region is often referred to as the "fingerprint region" because it contains characteristic absorption bands for various functional groups. Focus on identifying prominent peaks within this range. Key functional group absorptions include:
- O-H stretch (alcohols, carboxylic acids): Broad, strong peak around 3300-3600 cm⁻¹. The broadness is characteristic and indicative of hydrogen bonding. Carboxylic acids typically show a broader peak than alcohols.
- N-H stretch (amines, amides): Sharp peak around 3300-3500 cm⁻¹. Amines usually show multiple peaks in this region due to different vibrational modes.
- C-H stretch (alkanes, alkenes, alkynes): Sharp peaks around 2850-3000 cm⁻¹ (alkanes), 3000-3100 cm⁻¹ (alkenes), and 3300 cm⁻¹ (alkynes). The location of these peaks helps to distinguish between different types of C-H bonds.
- C≡N stretch (nitriles): Sharp, strong peak around 2200-2300 cm⁻¹.
- C=O stretch (aldehydes, ketones, carboxylic acids, esters, amides): Strong peak around 1650-1850 cm⁻¹. The exact location of this peak can vary significantly depending on the surrounding chemical environment. For example, esters tend to have higher frequency carbonyl stretches compared to ketones.
- C=C stretch (alkenes): Medium intensity peak around 1600-1680 cm⁻¹.
2. Analyze the Fingerprint Region (1500-400 cm⁻¹):
This region contains many complex absorptions that are specific to the molecule's overall structure. It is less useful for identifying functional groups but can help in distinguishing between isomers or closely related compounds. While individual peak assignments in this region are often challenging, comparing the fingerprint region of an unknown spectrum to a known spectrum can be crucial for confirming its identity.
3. Consider Peak Intensity and Shape:
The intensity of a peak is related to the number of bonds responsible for that absorption. Stronger peaks generally indicate a larger number of those specific bonds. The shape of the peak can also provide additional information. For instance, a broad peak might suggest hydrogen bonding, while a sharp peak suggests a less hindered bond.
4. Utilize Spectral Databases and Software:
Many online databases and software packages contain extensive spectral libraries. By comparing the unknown spectrum to those in the databases, you can often identify the compound or at least narrow down the possibilities.
Common Challenges in Interpreting IR Spectra
Even with experience, interpreting IR spectra can be challenging. Several factors can complicate the analysis:
- Overlapping peaks: Multiple absorptions may occur at similar wavenumbers, making it difficult to distinguish individual peaks. Spectral resolution can be crucial here.
- Weak or absent peaks: Some functional groups may exhibit weak or absent peaks, depending on their environment or the concentration of the sample.
- Solvent interference: Solvent molecules can absorb IR radiation, interfering with the absorption of the analyte. Choosing an appropriate solvent is essential for clean spectra.
- Sample preparation: Improper sample preparation can lead to broad, weak, or distorted peaks. The sample should be properly diluted and prepared to ensure accurate results.
Advanced Techniques and Considerations
Several advanced techniques can enhance the interpretation of IR spectra:
- Fourier Transform Infrared Spectroscopy (FTIR): This technique significantly improves the speed and sensitivity of IR measurements. FTIR is the standard technique used for most IR spectroscopic analysis.
- Attenuated Total Reflectance (ATR): ATR is a sampling technique that eliminates the need for sample preparation. It allows direct measurement of solid and liquid samples.
- Gas-phase IR spectroscopy: This technique is used to analyze gaseous samples and can provide valuable information about molecular structures and dynamics.
- Coupling with other techniques: Combining IR spectroscopy with other techniques like nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry (MS), and X-ray diffraction (XRD) provides a comprehensive characterization of the material.
Practical Tips for Effective Interpretation
- Start with the functional group region: Focus on identifying the major peaks in this region to narrow down the possibilities.
- Compare with known spectra: Consult spectral databases and literature to find similar spectra and compare their key features.
- Consider the chemical context: Take into account the expected functional groups based on the compound's synthesis or known properties.
- Use spectral interpretation software: Software can help with peak identification, deconvolution, and comparison with spectral databases.
- Be aware of limitations: Remember that IR spectroscopy may not provide sufficient information to fully characterize a complex molecule. Combining it with other techniques is often necessary.
Conclusion
Infrared spectroscopy is a valuable tool for identifying functional groups and characterizing molecules. Mastering the interpretation of IR spectra requires practice, a systematic approach, and a good understanding of fundamental principles. By following the steps outlined in this guide and utilizing available resources, you can significantly improve your ability to decipher IR spectra and unlock valuable information about the molecules you are studying. Remember to always critically analyze the entire spectrum, considering peak intensities, shapes, and the overall context to draw accurate conclusions. The journey to becoming proficient in IR spectral analysis is an iterative process, requiring continuous learning and practice. With dedication and effort, you can confidently interpret IR spectra and leverage this powerful technique in your research or analysis.
Latest Posts
Latest Posts
-
Ap Comparative Government And Politics Textbook
Mar 29, 2025
-
Use An Inverse Matrix To Solve The Linear System
Mar 29, 2025
-
Identifying Precipitation Combustion And Acid Base Reactions
Mar 29, 2025
-
Is Sweating A Negative Or Positive Feedback
Mar 29, 2025
-
According To Daltons Atomic Theory Atoms
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How To Read Ir Spectra Graphs . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
