Identifying Precipitation Combustion And Acid Base Reactions
Muz Play
Mar 29, 2025 · 7 min read
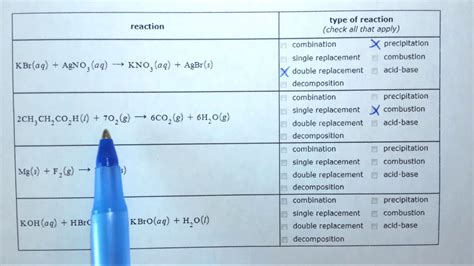
Table of Contents
Identifying Precipitation, Combustion, and Acid-Base Reactions
Chemical reactions are the backbone of chemistry, underpinning countless processes in our world, from the rusting of iron to the digestion of food. Understanding the different types of reactions is crucial for comprehending the behavior of matter and its transformations. This article will focus on three fundamental reaction types: precipitation reactions, combustion reactions, and acid-base reactions. We'll delve into their characteristics, identification methods, and real-world applications.
Precipitation Reactions: A Cloudy Affair
Precipitation reactions, also known as double displacement reactions, occur when two aqueous solutions containing soluble salts are mixed, resulting in the formation of an insoluble solid, known as a precipitate. The driving force behind this reaction is the formation of this insoluble compound. This insoluble product separates from the solution, typically appearing as a cloudy substance or a solid that settles at the bottom of the container.
Identifying Precipitation Reactions: Clues and Indicators
Identifying a precipitation reaction hinges on recognizing the formation of a precipitate. Several key indicators point towards this type of reaction:
-
Cloudiness or turbidity: The most obvious sign is the appearance of cloudiness in the initially clear solution. This indicates the formation of a solid precipitate.
-
Solid formation: A solid settling at the bottom of the container is another clear indication. This precipitate can vary in color, texture, and density depending on the specific reactants.
-
Color change: While not always present, a color change can sometimes accompany precipitate formation. This change reflects the different colors of the reactants and the newly formed solid.
-
Knowing solubility rules: Familiarity with solubility rules is critical. These rules predict whether a compound is soluble (dissolves in water) or insoluble (does not dissolve in water). Consult a solubility chart to determine if the possible products of a reaction are soluble or insoluble. If an insoluble product is predicted, a precipitation reaction is likely.
Examples of Precipitation Reactions
Several everyday examples highlight the occurrence of precipitation reactions:
-
Formation of limestone: The formation of limestone (calcium carbonate) in caves is a natural precipitation reaction. This process involves the reaction of calcium ions (Ca²⁺) and carbonate ions (CO₃²⁻) dissolved in groundwater, leading to the precipitation of insoluble calcium carbonate.
-
Water purification: Many water purification processes utilize precipitation reactions to remove heavy metal ions or other impurities. Reagents are added to the water to form insoluble precipitates containing these contaminants. These precipitates are then separated from the water, leaving behind cleaner water.
-
Silver halide photography: Traditional black-and-white photography relies on the precipitation of silver halides (like silver bromide, AgBr). Exposure to light triggers the reduction of silver ions, forming metallic silver and creating the photographic image.
Combustion Reactions: Fueling the Fire
Combustion reactions are exothermic redox reactions (oxidation-reduction) involving the rapid reaction of a substance with an oxidant, typically oxygen, producing heat and light. These reactions are characterized by their release of energy in the form of heat and often a visible flame. The fundamental process involves the oxidation of a fuel, resulting in the formation of oxidized products like carbon dioxide and water.
Identifying Combustion Reactions: The hallmarks of fire
Identifying combustion reactions is often straightforward due to their distinctive characteristics:
-
Presence of a flame: A visible flame is the most prominent indicator. The color and intensity of the flame can vary depending on the fuel and the conditions.
-
Heat generation: A significant release of heat is another hallmark. The heat generated can be intense enough to cause a rise in temperature.
-
Production of gases: Combustion often produces gaseous products, such as carbon dioxide (CO₂) and water vapor (H₂O). These gases can be observed as smoke or steam.
-
Rapid reaction: Combustion is typically a rapid reaction, often occurring in a short timeframe.
Examples of Combustion Reactions
Many everyday processes involve combustion reactions:
-
Burning of wood: The burning of wood in a fireplace or campfire is a classic example of combustion. Wood, primarily composed of cellulose and lignin, reacts with oxygen to produce carbon dioxide, water, and ash.
-
Internal combustion engines: Cars and other vehicles utilize combustion reactions in their internal combustion engines. The controlled burning of gasoline or diesel fuel in the engine's cylinders generates the energy that propels the vehicle.
-
Gas stoves: Gas stoves use the combustion of natural gas (primarily methane) to produce heat for cooking. The methane reacts with oxygen to form carbon dioxide and water.
-
Candle flames: A burning candle is a simple yet illustrative example of combustion. The wax, a hydrocarbon, reacts with oxygen in the air to produce carbon dioxide and water, generating heat and light.
Acid-Base Reactions: A Balancing Act
Acid-base reactions, also known as neutralization reactions, involve the transfer of protons (H⁺ ions) between an acid and a base. Acids are substances that donate protons, while bases are substances that accept protons. The reaction typically results in the formation of water and a salt. The defining characteristic of acid-base reactions is the neutralization of the acidic and basic properties of the reactants.
Identifying Acid-Base Reactions: The pH scale and indicators
Identifying acid-base reactions involves observing several key indicators:
-
pH changes: The most direct method involves measuring the pH of the solution before and after the reaction. A significant change in pH indicates an acid-base reaction. Acids have pH values less than 7, while bases have pH values greater than 7. Neutral solutions have a pH of 7.
-
Use of indicators: Acid-base indicators are substances that change color depending on the pH of the solution. These indicators can visually signal the occurrence of a neutralization reaction. Common indicators include litmus paper, phenolphthalein, and methyl orange.
-
Formation of salt and water: A characteristic outcome of acid-base reactions is the formation of water and a salt. The salt is an ionic compound formed from the cation of the base and the anion of the acid.
-
Heat generation: Many acid-base reactions are exothermic, meaning they release heat. This heat generation can be felt as a temperature increase.
Examples of Acid-Base Reactions
Numerous everyday applications involve acid-base reactions:
-
Digestion: The digestive system utilizes acid-base reactions to break down food. Stomach acid (hydrochloric acid) helps digest proteins, while the small intestine neutralizes the acidic chyme with bicarbonate ions.
-
Antacids: Antacids, used to relieve heartburn, neutralize excess stomach acid. They contain bases that react with hydrochloric acid to reduce acidity.
-
Soil pH adjustment: Farmers and gardeners often adjust soil pH using acids or bases to optimize plant growth. Different plants thrive in different pH ranges.
-
Soap making: Soap making involves an acid-base reaction between a fat (an ester) and a strong base (like sodium hydroxide). This process produces soap and glycerol.
Differentiating the Three Reaction Types
While precipitation, combustion, and acid-base reactions are distinct, some reactions might exhibit characteristics of more than one type. Careful observation and analysis are essential for correct classification. Here's a table summarizing the key differences:
| Feature | Precipitation Reaction | Combustion Reaction | Acid-Base Reaction |
|---|---|---|---|
| Reactants | Two aqueous solutions of soluble salts | Fuel and oxidant (usually oxygen) | Acid and base |
| Products | Insoluble precipitate and soluble salt | Carbon dioxide, water, and other oxides | Salt and water |
| Key Indicator | Formation of a precipitate | Flame, heat, and often gas production | pH change, heat generation, formation of salt and water |
| Energy Change | Usually little or no significant energy change | Always exothermic (releases heat) | Can be exothermic or endothermic |
| Example | Silver nitrate + sodium chloride → silver chloride (precipitate) + sodium nitrate | Burning of methane (CH₄) + Oxygen (O₂) → Carbon Dioxide (CO₂) + Water (H₂O) | Hydrochloric acid (HCl) + Sodium hydroxide (NaOH) → Sodium chloride (NaCl) + Water (H₂O) |
Conclusion: Mastering the Fundamentals
Understanding precipitation, combustion, and acid-base reactions is fundamental to grasping the principles of chemistry. By learning to identify these reactions through their characteristic features and employing appropriate analytical techniques, one can better comprehend the diverse chemical processes occurring around us. This knowledge forms a solid base for exploring more complex chemical phenomena and appreciating the intricate interplay of chemical reactions in our world. From the formation of rocks to the functioning of our bodies, these fundamental reactions are the building blocks of our physical reality. Continual practice in identifying these reactions through observation and experimentation will solidify your understanding and enhance your problem-solving skills in chemistry.
Latest Posts
Latest Posts
-
What Is The Difference Between Density Dependent And Density Independent
Mar 31, 2025
-
10 Common Diseases That Can Cause A Secondary Immunodeficiency
Mar 31, 2025
-
Factoring Trinomials With A Leading Coefficient
Mar 31, 2025
-
What Is The Coefficient Of Restitution
Mar 31, 2025
-
What Are The Basic Units Of All Living Things
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Identifying Precipitation Combustion And Acid Base Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
