How To Write A Rate Law For A Reaction
Muz Play
Apr 01, 2025 · 7 min read
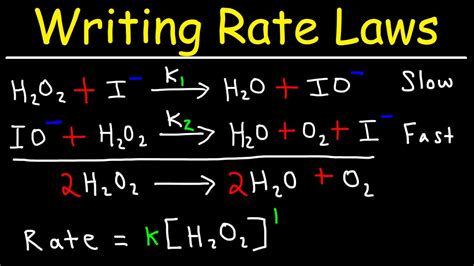
Table of Contents
How to Write a Rate Law for a Reaction: A Comprehensive Guide
Determining the rate law for a chemical reaction is a cornerstone of chemical kinetics. It allows us to understand and predict how reaction rates change with changes in reactant concentrations, temperature, and even the presence of catalysts. This comprehensive guide will walk you through the process, explaining the concepts, methods, and considerations involved in writing a rate law.
Understanding the Basics: Rate, Rate Constant, and Order
Before diving into the process, let's define some fundamental terms:
-
Rate of Reaction: This quantifies how quickly reactants are consumed or products are formed. It's typically expressed as the change in concentration (mol/L) per unit time (s, min, etc.).
-
Rate Constant (k): This is a proportionality constant specific to each reaction at a given temperature. It reflects the intrinsic reactivity of the reaction. Its value is independent of concentration but dependent on temperature.
-
Order of Reaction: This describes how the rate of reaction changes with the concentration of each reactant. It's determined experimentally and can be zero, positive, negative, or fractional. A reaction can have different orders with respect to different reactants. For example, a reaction might be first-order with respect to reactant A and second-order with respect to reactant B. The overall order of the reaction is the sum of the individual orders.
Experimental Methods for Determining Rate Laws
The rate law cannot be determined simply by looking at the balanced chemical equation. It must be determined experimentally. Here are the common methods:
1. Initial Rates Method
This is the most common method. It involves measuring the initial rate of the reaction at different starting concentrations of reactants, while keeping other factors constant (temperature, presence of a catalyst). By comparing the initial rates at varying concentrations, we can determine the order of the reaction with respect to each reactant.
Example: Consider a reaction: A + B → Products
Let's say we perform three experiments:
| Experiment | [A] (M) | [B] (M) | Initial Rate (M/s) |
|---|---|---|---|
| 1 | 0.1 | 0.1 | 0.005 |
| 2 | 0.2 | 0.1 | 0.020 |
| 3 | 0.1 | 0.2 | 0.010 |
Analysis:
-
Order with respect to A: Comparing experiments 1 and 2 (keeping [B] constant), we double [A] and the rate increases by a factor of 4 (0.020/0.005 = 4). This indicates that the reaction is second-order with respect to A (because 2² = 4).
-
Order with respect to B: Comparing experiments 1 and 3 (keeping [A] constant), we double [B] and the rate doubles (0.010/0.005 = 2). This indicates that the reaction is first-order with respect to B.
Therefore, the rate law is: Rate = k[A]²[B]
The overall order of the reaction is 2 + 1 = 3 (third-order).
2. Graphical Method
This method is particularly useful when the reaction follows simple kinetics (first-order or second-order). By plotting the appropriate data, we can determine the order and the rate constant.
-
First-order reaction: A plot of ln[A] vs. time yields a straight line with a slope of -k.
-
Second-order reaction: A plot of 1/[A] vs. time yields a straight line with a slope of k.
-
Zero-order reaction: A plot of [A] vs. time yields a straight line with a slope of -k.
3. Integrated Rate Laws
Integrated rate laws are mathematical expressions derived from the differential rate laws. They relate the concentration of a reactant to time. These can be used to determine the order and the rate constant, especially when analyzing data over a longer time period, as opposed to just the initial rate. The choice of which integrated rate law to use depends on the order of the reaction.
Writing the Rate Law: A Step-by-Step Approach
-
Identify the Reactants: Clearly identify all the reactants involved in the reaction.
-
Conduct Experiments: Perform several experiments, varying the initial concentrations of each reactant while keeping other factors constant (temperature, pressure, solvent, etc.). Measure the initial rate of reaction for each experiment. The more data points you have, the more accurate your determination of the rate law will be.
-
Determine the Order with Respect to Each Reactant: Analyze the experimental data using the initial rates method or the graphical method. This will determine the order (exponent) of each reactant in the rate law.
-
Write the Rate Law: Combine the rate constant (k) with the concentration terms for each reactant, raised to the power of their respective orders. The general form is:
Rate = k[A]^m[B]^n[C]^p...
where:
- k is the rate constant
- [A], [B], [C]… are the concentrations of the reactants
- m, n, p… are the orders of the reaction with respect to each reactant.
-
Determine the Overall Order: Sum the individual orders (m + n + p +…) to find the overall order of the reaction.
-
Calculate the Rate Constant (k): Use the data from one of your experiments and the rate law you derived to solve for k. Make sure to use the correct units for k that match the rate and concentration units used in your experiments.
Factors Affecting the Rate Law
Several factors can influence the rate law, even beyond the concentration of reactants:
-
Temperature: Increasing the temperature generally increases the rate constant (k) and thus the overall reaction rate. The Arrhenius equation quantifies this relationship.
-
Catalysts: Catalysts increase the rate of reaction without being consumed themselves. They provide an alternative reaction pathway with a lower activation energy, leading to a faster rate but do not change the overall order of the reaction. They may appear in the mechanism but not in the final rate law.
-
Solvent: The solvent can affect the rate of reaction through solvation effects on the reactants and intermediates.
-
Ionic Strength: In reactions involving ions, the ionic strength of the solution can influence the rate.
-
Surface Area: For heterogeneous reactions (reactions occurring at a surface), the surface area of the solid reactant can significantly affect the reaction rate.
Advanced Considerations: Complex Reactions
Not all reactions follow simple rate laws. Complex reactions, such as those involving multiple steps or intermediates, may have rate laws that are more complex and difficult to determine experimentally. In these cases, reaction mechanisms become crucial to understanding the rate law.
-
Rate-determining step: For complex reactions, one elementary step is usually much slower than the others and dictates the overall reaction rate. This slowest step is called the rate-determining step. The rate law is typically derived from the rate-determining step.
-
Steady-state approximation: This approximation simplifies the analysis of complex reactions by assuming that the concentration of intermediates remains relatively constant throughout the reaction.
-
Pre-equilibrium approximation: This approximation assumes that a fast equilibrium is established between reactants and intermediates before the rate-determining step occurs.
Example of a complex reaction with a rate-determining step:
Consider a reaction that occurs in two steps:
Step 1: A + B ⇌ C (fast equilibrium) Step 2: C + D → Products (slow, rate-determining)
The rate law for the overall reaction is determined by the rate-determining step:
Rate = k₂[C][D]
However, [C] is an intermediate, so we need to express it in terms of reactants. Since step 1 is a fast equilibrium, we can write the equilibrium constant:
K = [C]/([A][B])
Therefore, [C] = K[A][B]
Substituting this into the rate law for step 2, we get:
Rate = k₂K[A][B][D]
This illustrates that even though the reaction mechanism is multi-step, the overall rate law can be expressed in terms of reactant concentrations.
Conclusion
Determining and understanding the rate law for a chemical reaction is crucial for predicting reaction behavior and optimizing reaction conditions. While the initial rates method is a straightforward approach for simple reactions, more advanced techniques and considerations are necessary for complex reactions. Mastering these concepts forms a robust foundation for further studies in chemical kinetics and reaction engineering. Remember to always prioritize careful experimental design and thorough data analysis for accurate rate law determination.
Latest Posts
Latest Posts
-
Thesis Statement For Narrative Essay Example
Apr 02, 2025
-
Inborn Nonspecific Defenses Include And Barriers
Apr 02, 2025
-
Jewish Murals From The First Century Ce Depict
Apr 02, 2025
-
Which Quadratic Function Is Represented By The Graph
Apr 02, 2025
-
An Organized Arrangement Of Elements According To Their Atomic Number
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How To Write A Rate Law For A Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
