Ice Melting Is A Chemical Change
Muz Play
Mar 27, 2025 · 5 min read
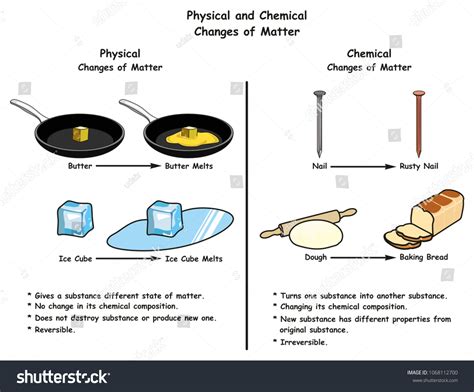
Table of Contents
- Ice Melting Is A Chemical Change
- Table of Contents
- Is Melting Ice a Chemical Change or a Physical Change? A Deep Dive
- Understanding Physical and Chemical Changes
- The Molecular Structure of Water: H₂O
- The Process of Melting Ice: A Physical Transformation
- Misconceptions and Clarifications
- The Role of Hydrogen Bonds in Melting Ice
- Exploring Further: Sublimation and Deposition
- Practical Applications and Real-World Examples
- Conclusion: Melting Ice Remains a Physical Change
- Latest Posts
- Latest Posts
- Related Post
Is Melting Ice a Chemical Change or a Physical Change? A Deep Dive
The question of whether melting ice is a chemical or physical change is a surprisingly common one, often sparking debate among students and science enthusiasts alike. The short answer is: melting ice is a physical change. However, understanding why requires a deeper look into the nature of matter, phase transitions, and the subtle distinctions between chemical and physical processes. This comprehensive article will explore this topic in detail, dispelling common misconceptions and providing a robust understanding of the science behind melting ice.
Understanding Physical and Chemical Changes
Before we delve into the specifics of ice melting, let's establish a clear definition of physical and chemical changes.
-
Physical Changes: These changes alter the form or appearance of a substance but do not change its chemical composition. The substance remains the same at a molecular level. Examples include changes in state (melting, freezing, boiling, condensation, sublimation), dissolving, and cutting. No new substance is formed.
-
Chemical Changes: These changes involve the rearrangement of atoms and molecules, resulting in the formation of new substances with different properties. These changes are often irreversible and are accompanied by changes in energy (heat, light, etc.). Examples include burning, rusting, cooking, and digestion. The chemical composition of the substance fundamentally alters.
The Molecular Structure of Water: H₂O
Water, in all its phases (solid, liquid, gas), is composed of the same molecule: H₂O. Each molecule consists of two hydrogen atoms covalently bonded to one oxygen atom. This is a crucial aspect to understanding why melting ice is a physical change. The chemical bonds within the water molecule itself remain intact throughout the entire process.
The Process of Melting Ice: A Physical Transformation
When ice (solid water) melts, it undergoes a phase transition from a solid to a liquid. This transformation is driven by an increase in temperature. As heat is absorbed by the ice, the kinetic energy of the water molecules increases.
-
Solid State (Ice): In ice, water molecules are arranged in a highly ordered crystalline structure, held together by relatively weak hydrogen bonds. These bonds restrict the movement of the molecules, resulting in a rigid, solid structure.
-
Liquid State (Water): As heat is applied, the kinetic energy of the water molecules overcomes the hydrogen bonds, causing the crystalline structure to break down. The molecules become more mobile and can move more freely, resulting in the liquid state.
-
Key Point: Throughout the melting process, the H₂O molecules themselves remain unchanged. There's no breaking or formation of covalent bonds between hydrogen and oxygen atoms. Only the arrangement and interaction between the molecules change. This is the defining characteristic of a physical change.
Misconceptions and Clarifications
Several misconceptions often arise concerning the melting of ice:
-
Myth 1: A change in state always implies a chemical change. This is incorrect. Phase transitions, like melting and boiling, are classic examples of physical changes. The chemical identity of the substance remains the same.
-
Myth 2: The appearance of liquid water is drastically different from ice, therefore it must be a chemical change. While the visual difference between ice and liquid water is significant (solid vs. liquid), this visual change doesn't equate to a chemical change. The underlying chemical composition remains unchanged.
-
Myth 3: Energy changes imply a chemical change. While chemical changes are often accompanied by energy changes (exothermic or endothermic), energy changes also occur during physical changes. Melting ice is an endothermic process (absorbs heat), but this energy is used to overcome intermolecular forces, not to break chemical bonds.
The Role of Hydrogen Bonds in Melting Ice
Hydrogen bonds play a pivotal role in the physical properties of water and explain many of its unique characteristics, including its relatively high melting and boiling points. These weak bonds between water molecules are responsible for the crystalline structure of ice and significantly influence the energy required for melting.
-
Strength of Hydrogen Bonds: Hydrogen bonds are considerably weaker than covalent bonds. They are easily broken and reformed, allowing for the fluidity of liquid water. This weakness ensures that melting ice is a relatively low-energy process, reinforcing its classification as a physical change.
-
Impact on Phase Transition Temperature: The strength of hydrogen bonds explains why water has a relatively high melting point compared to other substances with similar molecular weights. More energy is needed to overcome these bonds and transition to the liquid phase.
Exploring Further: Sublimation and Deposition
Water can also transition directly from solid (ice) to gas (water vapor) through a process called sublimation. Conversely, it can transition directly from gas to solid through deposition. Both of these processes are also classified as physical changes because they involve no alteration to the chemical composition of water. The water molecule remains H₂O throughout.
Practical Applications and Real-World Examples
Understanding the physical nature of ice melting has significant implications across various fields:
-
Glaciology: Studying the melting of glaciers and ice sheets is crucial for understanding climate change and its impact on sea levels. The processes involved are fundamentally physical changes in the state of water.
-
Cryopreservation: Freezing and thawing biological samples, a common practice in medicine and biology, relies on the reversible physical changes that water undergoes during phase transitions.
-
Food Science: Freezing and thawing food products are common preservation techniques. These processes involve physical changes in the water content of food, and understanding them is vital for ensuring food quality and safety.
-
Weather Forecasting: The formation of clouds, snow, and rain involves various phase transitions of water. Accurately modeling and predicting these weather phenomena relies on a thorough understanding of the physical changes involved.
Conclusion: Melting Ice Remains a Physical Change
In conclusion, melting ice is undeniably a physical change. While the macroscopic appearance changes dramatically from a solid to a liquid, the underlying chemical composition—H₂O—remains unaltered. The process involves breaking weak intermolecular forces (hydrogen bonds), not the strong covalent bonds within the water molecule. Understanding this fundamental distinction between physical and chemical changes is essential for grasping various scientific concepts across different disciplines. The seemingly simple act of ice melting holds a wealth of scientific information, highlighting the intricate interplay of energy, molecular structure, and phase transitions.
Latest Posts
Latest Posts
-
What Are Lone Pair Of Electrons
Mar 31, 2025
-
Definition Of Line In A Poem
Mar 31, 2025
-
Speed Of A Wave On A String
Mar 31, 2025
-
Is There Water In The Desert
Mar 31, 2025
-
Definition Of Marginal Analysis In Economics
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Ice Melting Is A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
