Identify The General Features Of An Amino Acid
Muz Play
Mar 25, 2025 · 7 min read
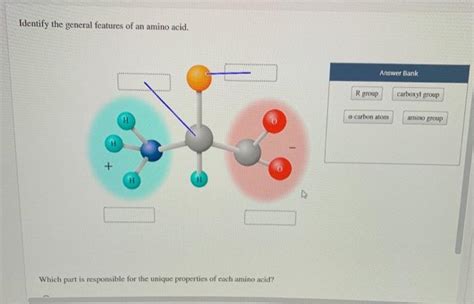
Table of Contents
Identifying the General Features of an Amino Acid: A Comprehensive Guide
Amino acids are the fundamental building blocks of proteins, essential molecules for life. Understanding their general features is crucial for comprehending biological processes at a molecular level. This comprehensive guide delves into the structure, properties, and classifications of amino acids, providing a detailed exploration of these vital biomolecules.
The Basic Structure: A Common Core with Unique Side Chains
At its core, every amino acid shares a common structural motif. This core consists of:
- A central carbon atom (α-carbon): This carbon atom is chiral (except for glycine), meaning it has four different groups attached to it. This chirality is vital for protein structure and function.
- A carboxyl group (-COOH): This acidic group contributes to the amino acid's overall charge and its ability to participate in hydrogen bonding. At physiological pH, this group is typically deprotonated, existing as -COO-.
- An amino group (-NH2): This basic group also contributes to the amino acid's charge and its capacity for hydrogen bonding. At physiological pH, it is typically protonated, existing as -NH3+.
- A hydrogen atom (-H): This completes the four groups attached to the central carbon atom.
- A variable side chain (R-group): This is the unique part of each amino acid, dictating its specific properties and influencing protein structure and function. It's the R-group that differentiates one amino acid from another.
This general formula can be represented as: H2N-CHR-COOH, where 'R' represents the side chain.
The Significance of the α-Carbon's Chirality
The chirality of the α-carbon (with the exception of glycine, which has two hydrogen atoms as its side chain) means that amino acids exist as stereoisomers, specifically enantiomers – mirror images that cannot be superimposed. In biological systems, almost all amino acids found in proteins are L-isomers. D-amino acids exist in nature, but their roles are generally different and often found in specific contexts like bacterial cell walls. The specific stereochemistry of amino acids is critical for proper protein folding and function. Incorrect stereochemistry can lead to dysfunctional proteins or even diseases.
Classifying Amino Acids Based on their R-Groups
Amino acids are categorized into groups based on the properties of their side chains (R-groups). These properties largely determine how the amino acid behaves within a protein and influences its overall structure and function.
1. Nonpolar, Aliphatic Amino Acids: Hydrophobic Interactions
These amino acids have nonpolar, hydrocarbon-based side chains. They tend to cluster together in the interior of proteins, away from the aqueous environment, due to hydrophobic interactions. Examples include:
- Glycine (Gly, G): The simplest amino acid, with a hydrogen atom as its side chain. It’s achiral.
- Alanine (Ala, A): Has a methyl group as its side chain.
- Valine (Val, V): Has a branched isopropyl group as its side chain.
- Leucine (Leu, L): Has a branched isobutyl group as its side chain.
- Isoleucine (Ile, I): Has a branched sec-butyl group as its side chain, another isomer of leucine.
- Methionine (Met, M): Contains a thioether group in its side chain.
These amino acids contribute to the hydrophobic core of proteins and play significant roles in protein stability.
2. Aromatic Amino Acids: Absorption of UV Light
These amino acids possess aromatic rings in their side chains. Their aromatic nature contributes to their hydrophobic character but also allows them to absorb ultraviolet (UV) light. This property is utilized in techniques like spectrophotometry to quantify protein concentrations. Examples include:
- Phenylalanine (Phe, F): Has a benzene ring as its side chain.
- Tyrosine (Tyr, Y): Has a benzene ring with a hydroxyl group.
- Tryptophan (Trp, W): Has an indole ring as its side chain.
The aromatic rings can participate in pi-stacking interactions, contributing to protein structure. Tyrosine's hydroxyl group can also participate in hydrogen bonding.
3. Polar, Uncharged Amino Acids: Hydrogen Bonding
These amino acids possess side chains with polar groups, capable of forming hydrogen bonds with water and other polar molecules. They are often found on the protein surface, interacting with the aqueous environment. Examples include:
- Serine (Ser, S): Has a hydroxyl group (-OH).
- Threonine (Thr, T): Has a hydroxyl group (-OH) attached to a chiral carbon.
- Cysteine (Cys, C): Contains a thiol group (-SH), which can form disulfide bonds with other cysteine residues.
- Asparagine (Asn, N): Has an amide group (-CONH2).
- Glutamine (Gln, Q): Has an amide group (-CONH2).
These amino acids play crucial roles in protein structure and function, often participating in enzyme active sites or mediating interactions with other molecules.
4. Positively Charged (Basic) Amino Acids: Attraction to Negatively Charged Molecules
These amino acids have side chains with positively charged groups at physiological pH. They are attracted to negatively charged molecules and play important roles in protein-protein interactions and enzyme catalysis. Examples include:
- Lysine (Lys, K): Has a terminal amino group (-NH3+).
- Arginine (Arg, R): Has a guanidinium group.
- Histidine (His, H): Has an imidazole group, which can be positively or neutrally charged depending on pH.
5. Negatively Charged (Acidic) Amino Acids: Repulsion to Other Negatively Charged Molecules
These amino acids possess side chains with negatively charged carboxyl groups at physiological pH. They repel other negatively charged molecules and contribute to protein stability through electrostatic interactions. Examples include:
- Aspartic acid (Asp, D): Has a carboxyl group (-COO-).
- Glutamic acid (Glu, E): Has a carboxyl group (-COO-).
These acidic amino acids often participate in enzyme catalysis or protein-protein interactions involving charge complementarity.
Amino Acid Properties and their Influence on Protein Structure
The properties of amino acid side chains directly impact the three-dimensional structure of proteins. These structures, which can range from simple to incredibly complex, are essential for protein function.
- Primary structure: This refers to the linear sequence of amino acids linked together by peptide bonds. The sequence is dictated by the genetic code.
- Secondary structure: This involves local folding patterns, such as α-helices and β-sheets, stabilized by hydrogen bonds between the backbone amide and carbonyl groups. The side chains play a less direct role here, but their size and polarity can influence the formation of secondary structures. For example, proline's rigid ring structure disrupts α-helices.
- Tertiary structure: This refers to the overall three-dimensional arrangement of a polypeptide chain. It’s stabilized by a multitude of interactions, including hydrophobic interactions, hydrogen bonds, disulfide bonds (between cysteine residues), and ionic interactions between charged side chains. The side chain properties are crucial in determining tertiary structure.
- Quaternary structure: This applies to proteins consisting of multiple polypeptide chains (subunits). The interactions between the subunits, again determined by side chain interactions, define the quaternary structure.
Post-Translational Modifications: Expanding Amino Acid Functionality
After protein synthesis, amino acids can undergo post-translational modifications. These modifications alter the properties of the amino acid side chains and can significantly impact the protein's function. Common modifications include:
- Phosphorylation: Addition of a phosphate group, often to serine, threonine, or tyrosine residues. This alters the charge and can activate or deactivate enzymes.
- Glycosylation: Attachment of carbohydrate groups, often to asparagine, serine, or threonine residues. This affects protein solubility and interactions.
- Acetylation: Addition of an acetyl group, often to the N-terminus of a protein. This can affect protein stability and interactions.
- Methylation: Addition of a methyl group, often to lysine or arginine residues. This influences protein-protein interactions and gene expression.
The Importance of Amino Acids in Human Health
Amino acids are not just building blocks; they play critical roles in various physiological processes:
- Enzyme catalysis: Many enzymes require specific amino acids in their active sites for catalytic activity.
- Hormone synthesis: Several hormones, like insulin and glucagon, are peptides composed of amino acids.
- Neurotransmitter synthesis: Neurotransmitters, like serotonin and dopamine, are derived from amino acids.
- Immune function: Antibodies are proteins composed of amino acids, vital for immune defense.
- Muscle growth and repair: Amino acids are essential for building and repairing muscle tissue.
- DNA and RNA synthesis: Amino acids are involved in the synthesis of nucleic acids.
Essential and Non-Essential Amino Acids: Dietary Considerations
Amino acids are classified as essential or non-essential based on whether the body can synthesize them.
- Essential amino acids: These must be obtained from the diet because the body cannot synthesize them in sufficient quantities. Examples include phenylalanine, valine, threonine, tryptophan, isoleucine, methionine, leucine, lysine, and histidine.
- Non-essential amino acids: These can be synthesized by the body from other metabolites. Examples include alanine, aspartic acid, asparagine, glutamic acid, glutamine, glycine, proline, serine, cysteine, and tyrosine.
Understanding the different types of amino acids and their properties is crucial for comprehending protein structure, function, and biological processes. This knowledge is vital in various fields, including medicine, biochemistry, and biotechnology. The continuing research into amino acids and their roles in health and disease promises exciting new discoveries and advancements in the future.
Latest Posts
Latest Posts
-
The Diels Alder Reaction Is A Concerted Reaction Define Concerted
Mar 26, 2025
-
How Many Fatty Acids Are Needed To Form A Glycerophospholipid
Mar 26, 2025
-
What Type Of Distortion Does The Good Homolosine Preserve
Mar 26, 2025
-
Autotrophs Make Their Own Food Using Energy From
Mar 26, 2025
-
Leave As Is To A Writer
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Identify The General Features Of An Amino Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
