In A Polar Covalent Bond Electrons Are Shared
Muz Play
Mar 18, 2025 · 7 min read
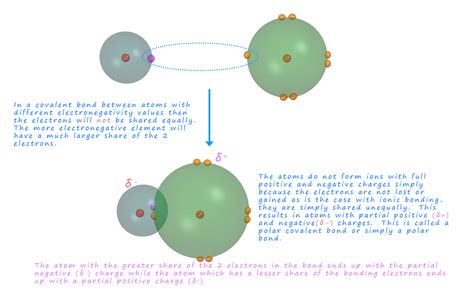
Table of Contents
In a Polar Covalent Bond, Electrons are Shared Unequally: A Deep Dive
Polar covalent bonds represent a fundamental concept in chemistry, crucial for understanding the properties of countless molecules and materials. Unlike nonpolar covalent bonds where electrons are shared equally between atoms, polar covalent bonds involve an unequal sharing of electrons due to differences in electronegativity. This unequal sharing creates a dipole moment, leading to regions of partial positive and partial negative charge within the molecule. Understanding this crucial difference is key to predicting molecular behavior, reactivity, and physical properties.
What is Electronegativity?
Before delving into the intricacies of polar covalent bonds, it's essential to grasp the concept of electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Elements with high electronegativity strongly attract electrons, while those with low electronegativity have a weaker pull. The periodic trend shows that electronegativity generally increases across a period (from left to right) and decreases down a group (from top to bottom). Fluorine (F) is the most electronegative element.
The Electronegativity Difference and Bond Type
The difference in electronegativity between two atoms determines the type of bond formed:
-
Nonpolar Covalent Bond: When the electronegativity difference is very small (typically less than 0.5), the electrons are shared almost equally between the atoms. This results in a nonpolar covalent bond, like the one found in diatomic molecules such as O₂ and Cl₂.
-
Polar Covalent Bond: When the electronegativity difference is significant (typically between 0.5 and 1.7), the electrons are shared unequally, creating a polar covalent bond. One atom attracts the electrons more strongly, resulting in a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the other atom. Examples include water (H₂O) and hydrogen chloride (HCl).
-
Ionic Bond: When the electronegativity difference is very large (typically greater than 1.7), one atom essentially steals the electron(s) from the other atom. This leads to the formation of ions (charged species) and an ionic bond. Examples include sodium chloride (NaCl) and magnesium oxide (MgO).
Understanding the Polar Covalent Bond: A Closer Look
In a polar covalent bond, the shared electrons spend more time closer to the more electronegative atom. This creates an uneven distribution of electron density, leading to the formation of a dipole. A dipole is a separation of positive and negative charges within a molecule. It's often represented by an arrow pointing from the partially positive atom (δ+) to the partially negative atom (δ-). The magnitude of the dipole moment is influenced by the electronegativity difference and the bond length.
Examples of Polar Covalent Bonds
Let's examine some common examples to solidify our understanding:
-
Water (H₂O): Oxygen is significantly more electronegative than hydrogen. The electrons in the O-H bonds are drawn closer to the oxygen atom, giving it a partial negative charge (δ-) and the hydrogen atoms partial positive charges (δ+). This polarity is crucial for water's unique properties, including its high boiling point, surface tension, and ability to act as a solvent.
-
Hydrogen Chloride (HCl): Chlorine is more electronegative than hydrogen. The electrons in the H-Cl bond are pulled more towards the chlorine atom, resulting in a partial negative charge (δ-) on the chlorine and a partial positive charge (δ+) on the hydrogen. This polarity contributes to HCl's reactivity and its ability to dissolve in water.
-
Carbon Monoxide (CO): Although carbon and oxygen are both nonmetals, oxygen's higher electronegativity makes this bond polar. Oxygen carries a partial negative charge, and carbon a partial positive charge. This polarity influences CO's reactivity and its interaction with other molecules.
Consequences of Polarity
The polarity of a molecule significantly affects its properties and behavior:
-
Solubility: Polar molecules tend to dissolve readily in polar solvents (like water), while nonpolar molecules dissolve better in nonpolar solvents (like oil). This is due to the principle of "like dissolves like."
-
Boiling Point and Melting Point: Polar molecules generally have higher boiling and melting points than nonpolar molecules of comparable size and molecular weight. This is because the dipole-dipole interactions between polar molecules are stronger than the weak London dispersion forces between nonpolar molecules. Hydrogen bonding, a special type of dipole-dipole interaction, is particularly strong and significantly impacts boiling and melting points.
-
Surface Tension: Polar molecules often exhibit higher surface tension than nonpolar molecules. The attractive forces between polar molecules create a stronger "skin" at the surface of the liquid.
-
Reactivity: The partial charges in polar molecules make them more reactive than nonpolar molecules. The partially positive and negative regions can attract other molecules or ions, facilitating chemical reactions.
Distinguishing Between Polar and Nonpolar Molecules: Molecular Geometry
It's crucial to understand that the presence of polar bonds within a molecule doesn't automatically make the entire molecule polar. The molecular geometry plays a vital role. If the polar bonds are symmetrically arranged, their dipole moments can cancel each other out, resulting in a nonpolar molecule.
Examples of Molecules with Polar Bonds but Nonpolar Molecular Geometry:
-
Carbon Dioxide (CO₂): Each C=O bond is polar, with oxygen being more electronegative. However, the linear geometry of CO₂ means the dipole moments of the two C=O bonds are equal in magnitude but opposite in direction. They cancel each other out, resulting in a nonpolar molecule.
-
Carbon Tetrachloride (CCl₄): The C-Cl bonds are polar due to the electronegativity difference between carbon and chlorine. However, the tetrahedral geometry of CCl₄ ensures that the dipole moments of the four C-Cl bonds cancel each other out, leading to a nonpolar molecule.
Applications of Polar Covalent Bonds
The concept of polar covalent bonds is fundamental to numerous applications in science and technology:
-
Drug Design: Understanding the polarity of molecules is crucial in drug design. The polarity of a drug molecule determines its solubility, absorption, distribution, and metabolism within the body. Polar drugs typically dissolve better in the bloodstream and are more easily transported to target sites.
-
Materials Science: The properties of many materials are heavily influenced by the polarity of their constituent molecules. Polar molecules are often used in the synthesis of polymers, liquid crystals, and other materials with specific desired properties.
-
Environmental Science: The polarity of pollutants influences their behavior in the environment, including their solubility in water, their ability to adsorb to soil particles, and their transport in air and water systems.
-
Biochemistry: Polar covalent bonds are essential to the structure and function of biological molecules such as proteins, nucleic acids, and carbohydrates. The interactions between polar groups in these molecules contribute to their three-dimensional structures and their ability to perform specific functions.
Advanced Concepts: Resonance and Bond Order
In certain molecules, the concept of polar covalent bonding becomes more nuanced.
Resonance Structures:
Some molecules exhibit resonance, where the actual electron distribution is a hybrid of multiple possible Lewis structures. Benzene (C₆H₆) is a classic example. While individual resonance structures show alternating single and double bonds, the actual electron distribution is a delocalized cloud above and below the plane of the ring, resulting in an intermediate bond order between a single and a double bond. Though each C-C bond is polar in individual resonance structures, the overall molecule is non-polar due to the symmetry and delocalization.
Bond Order and Bond Length:
Bond order reflects the number of electron pairs shared between two atoms. A higher bond order corresponds to a shorter and stronger bond. In polar bonds, the uneven electron distribution can affect both bond order and length. The atom with the greater electronegativity pulls the electrons closer, potentially leading to a slightly shorter bond length, compared to a similar nonpolar bond.
Conclusion: The Importance of Understanding Polar Covalent Bonds
In summary, understanding polar covalent bonds is fundamental to grasping the behavior and properties of countless molecules. The unequal sharing of electrons, driven by electronegativity differences, results in a dipole moment, which has profound consequences for a molecule's solubility, reactivity, boiling point, and more. By considering both the presence of polar bonds and the molecule's geometry, we can accurately predict and interpret its behavior, opening up a wealth of possibilities in various scientific fields. The concept extends beyond basic chemistry, impacting areas like material science, biochemistry, and environmental studies, highlighting its central role in understanding the natural world and developing innovative technologies. Furthermore, delving into advanced concepts like resonance and bond order provides a more complete picture of the intricate nature of chemical bonding and its implications.
Latest Posts
Latest Posts
-
Investigation Dna Proteins And Sickle Cell Answer Key
Mar 19, 2025
-
Buffer Region On A Titration Curve
Mar 19, 2025
-
Milk Of Magnesia Is Acidic Or Basic
Mar 19, 2025
-
How Does Melting Point Determine Purity
Mar 19, 2025
-
How To Calculate Percent Of Water In A Hydrate
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about In A Polar Covalent Bond Electrons Are Shared . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
