Ions With A Positive Charge Are Called
Muz Play
Mar 30, 2025 · 6 min read
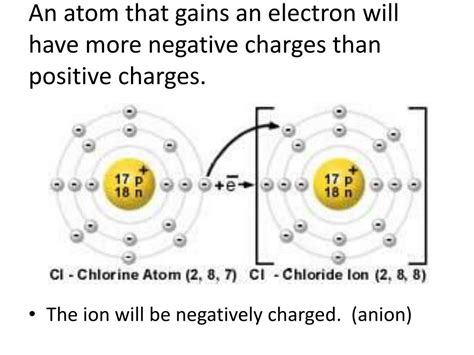
Table of Contents
Ions with a Positive Charge Are Called Cations: A Deep Dive into Ionic Chemistry
Ions are atoms or molecules that have gained or lost one or more electrons, resulting in a net electrical charge. Those with a positive charge are specifically called cations, while those with a negative charge are called anions. Understanding cations is crucial for comprehending a wide range of chemical processes, from the formation of ionic compounds to the functioning of biological systems. This article will delve into the fascinating world of cations, exploring their formation, properties, and significance across diverse scientific fields.
Understanding the Formation of Cations
The formation of a cation fundamentally involves the loss of one or more electrons from a neutral atom. This loss typically occurs when an atom interacts with another atom or molecule that has a higher electronegativity—a measure of an atom's ability to attract electrons in a chemical bond. Atoms with relatively low ionization energies (the energy required to remove an electron) readily form cations.
The Role of Electronegativity
Electronegativity plays a pivotal role in cation formation. Highly electronegative atoms, such as those in the halogens (fluorine, chlorine, bromine, iodine), strongly attract electrons. When these atoms interact with atoms of lower electronegativity, like alkali metals (lithium, sodium, potassium) or alkaline earth metals (magnesium, calcium, strontium), they can pull electrons away, leaving the latter atoms with a positive charge and thus forming cations.
Ionization Energy and Cation Formation
The ionization energy is a key determinant of how easily an atom forms a cation. Atoms with lower ionization energies require less energy to lose an electron and therefore are more likely to form cations. This property is related to the atomic structure, specifically the number of electrons in the outermost shell (valence electrons) and the effective nuclear charge experienced by these electrons. Atoms with loosely held valence electrons have lower ionization energies and tend to readily lose electrons, forming stable cations.
Examples of Cation Formation
Let's examine some specific examples:
-
Sodium (Na) forming Na⁺: Sodium has one valence electron. Losing this electron results in a stable electron configuration similar to neon (a noble gas), making it energetically favorable to form a Na⁺ cation.
-
Magnesium (Mg) forming Mg²⁺: Magnesium has two valence electrons. Losing both electrons results in a stable electron configuration similar to neon, leading to the formation of a Mg²⁺ cation.
-
Aluminum (Al) forming Al³⁺: Aluminum has three valence electrons. It loses all three to achieve a stable configuration, resulting in the Al³⁺ cation.
These examples highlight the general trend: atoms tend to lose electrons to achieve a stable, noble gas-like electron configuration, a principle often referred to as the octet rule.
Properties of Cations
Cations possess unique properties that distinguish them from neutral atoms and anions. These properties are primarily determined by their charge and size.
Charge and Ionic Radius
The positive charge of a cation is a fundamental characteristic. The magnitude of this charge depends on the number of electrons lost. For example, Na⁺ has a +1 charge, Mg²⁺ has a +2 charge, and Al³⁺ has a +3 charge. The charge significantly impacts the cation's interactions with other ions and molecules.
The ionic radius—the size of a cation—is smaller than its corresponding neutral atom. This is because the loss of electrons reduces electron-electron repulsion, allowing the remaining electrons to be drawn closer to the nucleus. This decrease in size influences the cation's reactivity and its ability to form chemical bonds.
Reactivity and Chemical Bonding
Cations are highly reactive, especially those with higher charges. Their positive charge attracts negatively charged ions (anions) and polar molecules, leading to the formation of ionic compounds and other types of interactions. The strength of these interactions depends on the charge and size of the cation. Smaller, highly charged cations generally form stronger interactions than larger, less charged cations.
Physical Properties
The physical properties of cations, such as melting point and boiling point, depend largely on the strength of the electrostatic forces between the cations and anions in an ionic compound. Compounds containing highly charged cations and small anions tend to have higher melting and boiling points due to stronger electrostatic attractions.
Significance of Cations in Different Fields
Cations play critical roles in various scientific fields, ranging from chemistry and materials science to biology and medicine.
Chemistry and Materials Science
Cations are fundamental building blocks of many inorganic materials. For example, ionic compounds like sodium chloride (NaCl) and calcium carbonate (CaCO₃) are composed of cations and anions held together by strong electrostatic forces. These compounds exhibit diverse properties depending on the specific cations and anions involved. Understanding the properties and interactions of cations is crucial for designing and developing new materials with specific functionalities. Many alloys and other materials also rely on cationic components for their desirable properties.
Biology and Medicine
Cations are essential for life. For instance, sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺) ions play crucial roles in many biological processes:
-
Sodium and Potassium Ions: These are vital for nerve impulse transmission and muscle contraction. The movement of these ions across cell membranes generates electrical signals that control these functions.
-
Calcium Ions: Calcium ions are involved in muscle contraction, blood clotting, and signal transduction pathways within cells.
-
Magnesium Ions: Magnesium ions are essential cofactors for many enzymes, which are biological catalysts that speed up chemical reactions in the body.
Imbalances in cation concentrations can lead to various health problems. For example, electrolyte imbalances, involving disruptions in sodium, potassium, and calcium levels, can have severe consequences. Maintaining the proper balance of these cations is critical for good health.
Environmental Science
Cations are significant in environmental science due to their roles in water quality and soil chemistry. The presence and concentration of specific cations in water sources affect water quality and the ability of aquatic life to thrive. Soil cation exchange capacity—the ability of soil to hold and release cations—is vital for plant growth and nutrient availability. Understanding cation behavior in environmental systems is critical for addressing issues related to water pollution, soil degradation, and environmental sustainability.
Examples of Specific Cations and Their Applications
Let's explore some specific examples of cations and their significant applications:
-
Sodium (Na⁺): Used extensively in table salt (NaCl), sodium hydroxide (NaOH) for various industrial applications, and in street lighting (sodium vapor lamps).
-
Potassium (K⁺): Essential for plant growth (potassium fertilizers), plays a crucial role in nerve impulse transmission in animals, and is found in various electrolytes.
-
Calcium (Ca²⁺): Found in bones and teeth, plays a key role in muscle contraction and blood clotting, and is used in cement and plaster.
-
Magnesium (Mg²⁺): A crucial cofactor in many enzymatic reactions, found in chlorophyll (essential for photosynthesis), and used in alloys (lightweight and strong).
-
Iron (Fe²⁺ and Fe³⁺): Crucial for oxygen transport in the blood (hemoglobin), used in steel production, and plays roles in various enzymatic processes.
Conclusion: The Importance of Understanding Cations
Cations are ubiquitous in the natural world and play essential roles in a wide range of chemical and biological processes. Understanding their formation, properties, and interactions is crucial for advancing our knowledge in numerous scientific fields. From the development of new materials to the understanding of fundamental biological mechanisms, the study of cations continues to yield significant insights and drive progress in diverse areas of scientific inquiry. Further research into the behavior and applications of cations will undoubtedly lead to even more significant advancements in the future. The continuing exploration of cationic chemistry offers a rich and promising path for future scientific discovery.
Latest Posts
Latest Posts
-
How To Name Ionic And Covalent Bonds
Apr 01, 2025
-
Liquid In A Liquid Solution Example
Apr 01, 2025
-
Express The Inequality Using Interval Notation
Apr 01, 2025
-
Difference Between Hypothesis Law And Theory
Apr 01, 2025
-
Is An Atom The Smallest Particle
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Ions With A Positive Charge Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
