Iron Rust Chemical Or Physical Change
Muz Play
Mar 22, 2025 · 6 min read
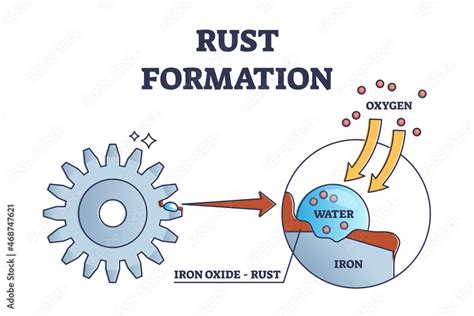
Table of Contents
Is Rusting Iron a Chemical or Physical Change? A Deep Dive into Oxidation
The question of whether rusting iron is a chemical or physical change is a fundamental concept in chemistry. While seemingly simple, understanding the process reveals a fascinating interplay of elements and reactions that go far beyond a simple surface-level observation. This comprehensive exploration delves into the intricacies of iron oxidation, definitively classifying it as a chemical change and exploring the scientific principles involved. We'll also examine the factors influencing the rate of rusting and discuss methods to prevent or mitigate this common phenomenon.
Understanding Chemical vs. Physical Changes
Before diving into the specifics of rusting, it's crucial to establish a clear understanding of the difference between chemical and physical changes.
Physical changes alter the form or appearance of a substance without changing its chemical composition. Examples include melting ice (water changes from solid to liquid), tearing paper, or dissolving sugar in water. The fundamental chemical structure of the substance remains unchanged. These changes are often reversible.
Chemical changes, also known as chemical reactions, involve a change in the chemical composition of a substance. New substances with different properties are formed. Examples include burning wood (wood transforms into ash and gases), cooking an egg (protein structure alters irreversibly), and the baking of a cake (ingredients react to create a new substance). These changes are usually irreversible.
The Rusting Process: A Chemical Reaction Unveiled
Rust, chemically known as iron(III) oxide, (Fe₂O₃), is the result of a complex chemical reaction called oxidation. This is not merely a change in appearance; it's a fundamental alteration of the iron's chemical structure.
Here's a breakdown of the process:
1. The Role of Oxygen:
Iron's inherent reactivity with oxygen is the primary driver of rust formation. In the presence of oxygen, iron atoms readily lose electrons (oxidation). This process transforms metallic iron (Fe) into iron ions (Fe²⁺ and Fe³⁺).
2. The Influence of Water:
Water acts as an electrolyte, facilitating the movement of electrons. It significantly accelerates the oxidation process. The presence of water allows for the formation of an electrolytic solution on the iron surface, speeding up the electron transfer between iron and oxygen.
3. The Formation of Iron Oxide:
The iron ions (Fe²⁺ and Fe³⁺) react with oxygen and water molecules to form hydrated iron oxides, which are the various forms of rust. The exact composition of the rust can vary depending on the conditions, leading to different colors and textures. Common forms include iron(II) oxide (FeO), iron(III) oxide (Fe₂O₃), and iron(II,III) oxide (Fe₃O₄).
4. The Electrochemical Nature of Rusting:
Rusting isn't simply a direct reaction; it's an electrochemical process. This means there are distinct anodic and cathodic sites on the iron surface. At the anode, iron loses electrons, forming iron ions and initiating the oxidation process. At the cathode, oxygen gains electrons, forming hydroxide ions. These reactions are linked by the flow of electrons through the iron and the electrolyte (water). This electrochemical mechanism explains why rusting is accelerated in the presence of electrolytes (such as salt water).
Evidence Supporting Rusting as a Chemical Change
Several key observations solidify the classification of rusting as a chemical change:
- Irreversibility: Once iron rusts, it's difficult, if not impossible, to revert it back to its original metallic state through simple physical means. The chemical composition has fundamentally changed.
- Change in Properties: Rust possesses entirely different properties from metallic iron. It's brittle, powdery, and lacks the conductivity and strength of its metallic precursor. This demonstrates a significant alteration in the material's physical and chemical characteristics.
- Formation of a New Substance: The creation of iron oxide (rust) is the formation of a distinctly different chemical compound. This new substance has its unique chemical formula and properties, completely distinct from elemental iron.
- Energy Changes: Rusting is an exothermic reaction, meaning it releases energy in the form of heat. While the heat released is subtle and not readily noticeable, this energy change is a hallmark of chemical reactions.
Factors Affecting the Rate of Rusting
The rate at which iron rusts isn't constant; it's influenced by several environmental factors:
- Presence of Water: Water is crucial for the electrochemical process of rusting. Dry iron will not rust significantly. Increased humidity accelerates rust formation.
- Presence of Oxygen: Oxygen is the primary oxidizing agent in rusting. A higher concentration of oxygen leads to faster rusting.
- Acidity (pH): Lower pH (more acidic conditions) speeds up the rusting process. Acidic solutions enhance the electrolyte's conductivity and accelerate the electrochemical reaction.
- Temperature: Higher temperatures generally increase the rate of chemical reactions, including rusting.
- Presence of Electrolytes: Electrolytes, such as salts, dissolved in water, significantly increase the conductivity of the solution, leading to faster rusting. This is why saltwater environments are particularly corrosive.
- Surface Area: A larger surface area of the iron exposed to the environment will increase the rate of rusting. This is why iron powder rusts faster than a solid iron bar of the same weight.
Preventing and Mitigating Rust
Given the detrimental effects of rust on iron structures and objects, understanding and implementing rust prevention techniques is crucial. Several methods effectively mitigate or prevent rust:
- Protective Coatings: Applying coatings like paint, varnish, or oil creates a barrier between the iron and the environment, preventing contact with oxygen and water.
- Galvanization: This process involves coating iron with a layer of zinc. Zinc is more reactive than iron, thus it oxidizes preferentially, protecting the iron underneath. This is a highly effective method used extensively in construction and manufacturing.
- Alloying: Creating iron alloys, such as stainless steel, incorporates other elements that increase corrosion resistance. The addition of chromium, for instance, forms a passive oxide layer that protects the underlying metal.
- Cathodic Protection: This electrochemical technique uses a sacrificial anode (a more reactive metal) to protect the iron. The sacrificial anode corrodes preferentially, preventing rust formation on the iron structure. This is commonly used for pipelines and underground storage tanks.
- Regular Cleaning and Maintenance: Regularly cleaning and removing existing rust can slow down further corrosion. Applying rust inhibitors can also help to prevent further rust formation.
Conclusion: Rusting – A Chemical Transformation
In conclusion, rusting iron is undeniably a chemical change. The transformation of iron into iron oxide involves a complex electrochemical reaction, resulting in the formation of a new substance with significantly different properties. Understanding the factors that influence the rate of rusting and the various prevention methods are crucial for preserving iron structures and extending their lifespan. The ongoing research into corrosion mechanisms continues to drive innovation in materials science and engineering, providing improved solutions for protecting iron and steel from this pervasive chemical phenomenon. The seemingly simple process of rusting provides a window into the broader world of chemical reactions and the essential role of oxidation in the natural world.
Latest Posts
Latest Posts
-
Is Childbirth Positive Or Negative Feedback
Mar 22, 2025
-
How Was The Modern Periodic Table Arranged
Mar 22, 2025
-
Openings That Allow For Gas Exchange
Mar 22, 2025
-
Determinants Of Price Elasticity Of Supply
Mar 22, 2025
-
What Is Stronger Ionic Or Covalent Bonds
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Iron Rust Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
