Is A Homogeneous Mixture A Solution
Muz Play
Mar 15, 2025 · 5 min read
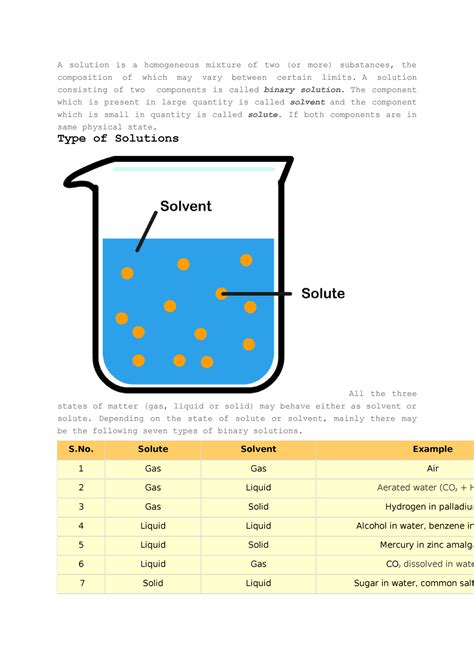
Table of Contents
Is a Homogeneous Mixture a Solution? Understanding the Relationship
The terms "homogeneous mixture" and "solution" are often used interchangeably, leading to confusion about their precise meanings and the subtle differences between them. While the relationship is close, they aren't perfectly synonymous. This comprehensive guide delves into the definitions of both, explores their similarities and differences, and provides examples to clarify the nuances involved. Understanding this distinction is crucial for anyone studying chemistry, materials science, or related fields.
Defining Homogeneous Mixtures
A homogeneous mixture is a type of mixture where the components are uniformly distributed throughout the mixture. This means that at a macroscopic level, the mixture appears to have a uniform composition and properties. No matter where you sample the mixture, you'll find the same proportions of the constituent components. Crucially, the individual components retain their chemical identities; they haven't undergone a chemical reaction to form a new substance.
Key Characteristics of Homogeneous Mixtures:
- Uniform Composition: The components are evenly distributed at the macroscopic level. You won't see visible separation or distinct layers.
- No Chemical Change: The components retain their original chemical properties.
- Single Phase: A homogeneous mixture exists in a single phase – either solid, liquid, or gas.
Defining Solutions
A solution is a special type of homogeneous mixture composed of two or more substances. One key distinguishing feature of a solution is that it consists of a solute, which is the substance being dissolved, and a solvent, which is the substance doing the dissolving. The solvent typically makes up the larger portion of the solution. Like homogeneous mixtures, solutions exhibit uniform composition at a macroscopic level.
Key Characteristics of Solutions:
- Homogeneous: The solute and solvent are uniformly dispersed.
- Solute-Solvent Interaction: The solute particles are completely surrounded by solvent molecules. This interaction is crucial for dissolution.
- Particle Size: Solute particles are incredibly small, typically at the atomic or molecular level. This ensures transparency (unless the solution is inherently colored by the solute).
- Filtration Inability: You can't separate the components of a solution using simple filtration techniques.
The Relationship Between Homogeneous Mixtures and Solutions
The crucial connection is this: all solutions are homogeneous mixtures, but not all homogeneous mixtures are solutions.
This statement highlights the hierarchical relationship. Solutions represent a more specific subcategory within the broader category of homogeneous mixtures. The key difference lies in the distinct roles of solute and solvent and the resulting intermolecular interactions.
Examples to Illustrate the Difference
Let's consider some examples to illuminate the distinctions:
Examples of Homogeneous Mixtures that are ALSO Solutions:
- Saltwater: Salt (NaCl) is the solute, and water (H₂O) is the solvent. The salt ions are uniformly dispersed among the water molecules.
- Sugar water: Sugar is the solute, and water is the solvent. Similar to saltwater, the sugar molecules are evenly distributed in the water.
- Air: A mixture of gases (primarily nitrogen and oxygen). Although the proportions might vary slightly depending on location, air is considered a homogeneous mixture and is often referred to as a solution of gases.
- Brass: An alloy of copper and zinc, uniformly mixed at the atomic level. While not explicitly defined as a solute and solvent, the components are uniformly distributed.
Examples of Homogeneous Mixtures that are NOT Solutions (at least not traditionally):
- Air (Again, but with a caveat): While we treat air as a solution of gases, the strict definition of a "solute" and "solvent" is less clear. We don't typically think of one gas dissolving into another in the same way that salt dissolves into water. It's a homogeneous mixture, undeniably, but the solute/solvent distinction blurs.
- Many Alloys: While brass is often considered a solution, many other alloys might exhibit a more complex microstructure than a simple solute/solvent mixture. The interactions between constituent metals could be more nuanced.
- Some Solid Solutions: Some homogenous solid mixtures are described as solid solutions, where one solid dissolves into another (e.g., certain metal alloys). However, the concept of "dissolving" in solids differs from the liquid-based dissolution in typical solutions.
Why the Distinction Matters
The distinction between a homogeneous mixture and a solution might seem pedantic, but it's important for several reasons:
- Precise Scientific Communication: Using the correct terminology ensures clear communication within the scientific community.
- Understanding Properties: The solute-solvent interaction in solutions influences properties like boiling point elevation, freezing point depression, and osmotic pressure. These properties aren't always as predictable or significant in general homogeneous mixtures.
- Predicting Behavior: Understanding the nature of the mixture helps predict its behavior under different conditions. For instance, knowing whether a mixture is a true solution aids in determining its solubility, reactivity, and other relevant properties.
- Applications in Various Fields: This distinction is critical in various fields, such as pharmaceutical science (drug solubility), materials science (alloy design), and environmental science (water pollution studies).
Beyond Simple Solutions: Colloids and Suspensions
To fully grasp the spectrum of mixtures, it’s essential to differentiate solutions from colloids and suspensions. These also exhibit different levels of homogeneity:
-
Colloids: Colloids are mixtures where particles are larger than those in solutions but smaller than those in suspensions. They are heterogeneous at the microscopic level but appear homogeneous at the macroscopic level. Examples include milk and fog. While uniformly distributed, colloids show the Tyndall effect (scattering of light).
-
Suspensions: Suspensions are heterogeneous mixtures with larger particles that settle out over time. Examples include muddy water or sand in water. These are clearly non-uniform in composition and require stirring to maintain a seeming homogeneity.
The comparison emphasizes the gradation in particle size influencing the mixture's homogeneity and behavior.
Conclusion: A Matter of Perspective and Definition
The relationship between homogeneous mixtures and solutions is a matter of perspective and precise definition. While all solutions are undoubtedly homogeneous mixtures, not all homogeneous mixtures fit the stricter criteria of a solution (especially with regards to the defined solute and solvent roles). This understanding is fundamental to comprehending the behavior and properties of various materials and mixtures in numerous scientific and engineering applications. By carefully distinguishing between these terms, we gain a more nuanced and accurate understanding of the world around us, composed as it is of diverse and fascinating mixtures.
Latest Posts
Latest Posts
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
-
Why Was The Discovery Of Noble Gases A Problem
Mar 17, 2025
-
Evidence Of Light As A Particle
Mar 17, 2025
-
Do Achiral Molecules Have A Plane Of Symmetry
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is A Homogeneous Mixture A Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
