Is A Phosphate Group Polar Or Nonpolar
Muz Play
Mar 28, 2025 · 5 min read
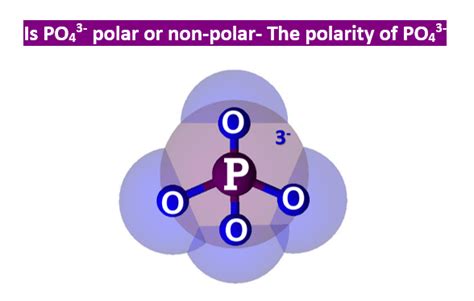
Table of Contents
Is a Phosphate Group Polar or Nonpolar? A Deep Dive into Molecular Polarity
The question of whether a phosphate group is polar or nonpolar is a fundamental one in biochemistry and chemistry. Understanding molecular polarity is crucial for predicting the behavior of molecules in various environments, including their solubility, interactions with other molecules, and their overall biological function. This article delves into the intricacies of phosphate group polarity, examining its structure, bond types, and the factors contributing to its overall polar nature. We’ll explore the implications of this polarity in biological systems and address common misconceptions.
Understanding Polarity: A Refresher
Before we dive into the specifics of the phosphate group, let's establish a clear understanding of molecular polarity. Polarity arises from the unequal distribution of electrons within a molecule. This unequal distribution is caused by differences in electronegativity between the atoms forming the molecule.
Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. Atoms with high electronegativity, such as oxygen and nitrogen, strongly attract electrons, while atoms with low electronegativity, such as hydrogen and carbon, attract electrons less strongly.
When a molecule has a significant difference in electronegativity between its atoms, the electrons are pulled more towards the more electronegative atom, creating a dipole moment. This dipole moment results in a partial negative charge (δ-) on the more electronegative atom and a partial positive charge (δ+) on the less electronegative atom. Molecules with significant dipole moments are considered polar. Conversely, molecules with symmetrical distribution of electrons and minimal differences in electronegativity are considered nonpolar.
The Structure of a Phosphate Group
The phosphate group (PO₄³⁻) is a crucial functional group in many biological molecules, including nucleotides (the building blocks of DNA and RNA), phospholipids (major components of cell membranes), and ATP (the energy currency of cells). Its structure consists of a central phosphorus atom covalently bonded to four oxygen atoms.
Three of these oxygen atoms are singly bonded to the phosphorus, each carrying a formal negative charge. The fourth oxygen atom is doubly bonded to the phosphorus. This arrangement gives rise to the tetrahedral geometry characteristic of phosphate groups. The presence of these negatively charged oxygen atoms is critical to understanding the phosphate group's polarity.
Why the Phosphate Group is Polar
Several factors contribute to the phosphate group's strong polarity:
1. High Electronegativity of Oxygen:
Oxygen is a highly electronegative atom. The oxygen atoms in the phosphate group strongly attract the shared electrons in the P-O bonds. This creates a significant difference in electron density between the phosphorus and oxygen atoms.
2. Multiple Polar Bonds:
The phosphate group contains multiple P-O bonds, each exhibiting a substantial dipole moment due to the electronegativity difference between phosphorus and oxygen. These individual dipole moments add up vectorially, resulting in a large overall dipole moment for the entire phosphate group. This significant dipole moment signifies a strongly polar nature.
3. Negative Charges:
The three negatively charged oxygen atoms further enhance the polarity of the phosphate group. These negative charges represent a significant accumulation of electron density on these atoms, leading to a substantial overall negative charge and further contributing to the strong dipole moment.
4. Tetrahedral Geometry:
While the individual bond dipoles might seem to cancel out in a perfectly symmetrical tetrahedral geometry, the presence of the three negatively charged oxygen atoms breaks this symmetry. This asymmetry reinforces the overall polarity of the molecule, preventing any cancellation of dipole moments.
Implications of Phosphate Group Polarity in Biological Systems
The polar nature of the phosphate group has profound consequences for its biological function and interactions:
-
Solubility: The phosphate group's polarity makes it highly soluble in water, a polar solvent. This solubility is crucial for its role in aqueous biological systems. Many biomolecules containing phosphate groups readily dissolve in the cellular environment.
-
Interactions with Other Molecules: The negative charges on the phosphate group allow it to form strong electrostatic interactions (ionic bonds) with positively charged molecules or regions of molecules. This property is essential for the interactions of nucleotides in DNA and RNA, as well as the binding of ATP to enzymes.
-
Energy Transfer: The high energy phosphate bonds in ATP are crucial for energy transfer in cells. The polarity and charge distribution within the phosphate group are key determinants of the high energy content of these bonds. The hydrolysis of these bonds releases energy for various cellular processes.
-
Membrane Interactions: Phospholipids, which contain phosphate groups in their polar head groups, are essential components of cell membranes. The polarity of the phosphate head group is crucial for their interaction with the aqueous environment and their arrangement in the lipid bilayer structure.
Addressing Common Misconceptions
-
Phosphate is not always highly polar: While the phosphate group is highly polar, its polarity can be influenced by its environment and the molecules it interacts with. The overall polarity of a larger molecule containing a phosphate group will depend on the other functional groups present and their distribution.
-
Polarity doesn't dictate all interactions: While polarity is a significant factor governing interactions, other factors such as hydrogen bonding, van der Waals forces, and hydrophobic effects also play critical roles in molecular interactions involving phosphate groups.
-
The term "polar" is relative: The degree of polarity can vary, and the phosphate group's strong polarity places it firmly in the category of strongly polar, yet some organic molecules could possess weaker polarity. The context within a molecule and relative comparisons are key to avoid misconceptions.
Conclusion
In conclusion, the phosphate group is unequivocally polar. Its high electronegativity oxygen atoms, multiple polar P-O bonds, three negative charges, and asymmetrical geometry all contribute to its significant dipole moment. This polarity is fundamental to its role in various biological systems, dictating its solubility, interactions with other molecules, and its participation in crucial processes like energy transfer and membrane structure. Understanding the phosphate group's polarity is therefore essential for comprehending the intricate workings of biological systems at the molecular level. Further research into the detailed dynamics of phosphate group interactions and their precise impact on various biological functions continues to be an active area of investigation. The knowledge presented here forms the foundational understanding needed to appreciate these ongoing advances.
Latest Posts
Latest Posts
-
What Is The Secondary Structure Of Dna
Mar 31, 2025
-
Is Water An Ionic Or Covalent Bond
Mar 31, 2025
-
Why Would A Beta Sheet Not Have Alternating Polar Nonpolar Aa
Mar 31, 2025
-
What Does A Chemical Equation Describe
Mar 31, 2025
-
How Do You Divide A Whole Number
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is A Phosphate Group Polar Or Nonpolar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
