Is Acid Catalyzed Hydration Syn Or Anti
Muz Play
Mar 27, 2025 · 5 min read
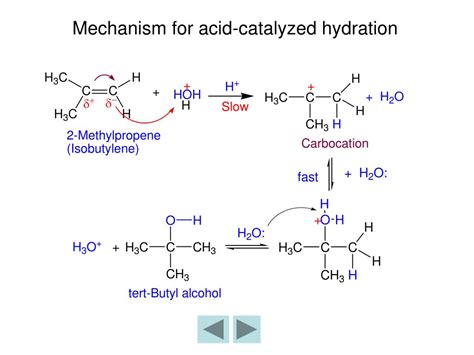
Table of Contents
Is Acid-Catalyzed Hydration Syn or Anti? A Deep Dive into Regio- and Stereoselectivity
The acid-catalyzed hydration of alkenes is a fundamental reaction in organic chemistry, transforming a carbon-carbon double bond into an alcohol functional group. Understanding the stereochemistry of this reaction – whether it proceeds via a syn or anti addition – is crucial for predicting the structure of the product and designing efficient synthetic routes. This detailed analysis will explore the mechanism, delve into the stereochemical implications, and address common misconceptions surrounding the syn vs. anti debate in acid-catalyzed alkene hydration.
The Mechanism: Unveiling the Stereochemical Fate
The acid-catalyzed hydration of alkenes follows a two-step mechanism involving a carbocation intermediate. Let's break it down:
Step 1: Protonation of the Alkene
The reaction begins with the protonation of the alkene by a strong acid, such as sulfuric acid (H₂SO₄) or phosphoric acid (H₃PO₄). The electrophilic proton attacks the carbon-carbon double bond, forming a more stable carbocation. This step is crucial in determining the regioselectivity of the reaction, meaning which carbon atom the hydroxyl group will eventually attach to. Markovnikov's rule dictates that the proton will add to the less substituted carbon, leading to the formation of the more substituted carbocation. This is due to the greater stability of the more substituted carbocation, which is stabilized by hyperconjugation.
Step 2: Nucleophilic Attack and Deprotonation
In the second step, a water molecule acts as a nucleophile, attacking the positively charged carbocation. This results in the formation of an oxonium ion. Finally, a base (often a water molecule or the conjugate base of the acid) abstracts a proton, generating the alcohol product and regenerating the acid catalyst.
The crucial point regarding stereochemistry lies in the nature of the carbocation intermediate. This intermediate is planar, meaning there's no inherent preference for the subsequent nucleophilic attack from one side or the other. The water molecule can attack from either the top or the bottom face of the planar carbocation with equal probability.
Stereochemical Implications: The Absence of Stereoselectivity
This lack of steric bias in the attack on the planar carbocation means that the acid-catalyzed hydration of alkenes is generally not stereospecific. It doesn't favor syn or anti addition. Instead, the product is a racemic mixture (a 50:50 mixture of enantiomers) if the carbocation is not chiral.
Consider this example: The acid-catalyzed hydration of propene. The secondary carbocation formed is achiral. Therefore, attack by water from either side generates a racemic mixture of 2-propanol enantiomers. No stereochemical preference exists.
Regioselectivity versus Stereoselectivity: A Clear Distinction
It's vital to differentiate between regioselectivity and stereoselectivity in this reaction.
-
Regioselectivity refers to the preference for the formation of one constitutional isomer over another. In acid-catalyzed hydration, Markovnikov's rule governs regioselectivity, favoring the more substituted alcohol.
-
Stereoselectivity refers to the preferential formation of one stereoisomer over another. In the case of acid-catalyzed alkene hydration, there's generally no stereoselectivity unless the carbocation is chiral.
Exceptions: Chiral Carbocations and Stereoselectivity
The statement that acid-catalyzed hydration is not stereospecific needs a crucial caveat: it only holds true if the carbocation intermediate is achiral. If the carbocation is chiral, then the nucleophilic attack will be influenced by steric factors, potentially leading to some degree of diastereoselectivity (although rarely complete stereoselectivity).
For instance: The acid-catalyzed hydration of a substituted alkene that forms a chiral carbocation may exhibit some diastereoselectivity, favoring one diastereomer over the other, due to steric hindrance or other factors. However, this is not the typical outcome.
Addressing Common Misconceptions
Several misconceptions often surround the stereochemistry of acid-catalyzed alkene hydration:
Misconception 1: Acid-catalyzed hydration is always syn addition.
Reality: This is incorrect. The planar nature of the carbocation intermediate allows for attack from either side, leading to a racemic mixture in most cases. Syn addition is only observed in specific circumstances involving specific reagents and reaction conditions, not in typical acid-catalyzed hydration.
Misconception 2: The reaction is anti addition because water attacks from the opposite side of the proton.
Reality: While the proton adds to one carbon and the hydroxyl group to the other, this doesn't inherently imply anti addition. The key is the intermediate's planar geometry, permitting attack from either face. The relative orientations of the additions are not determined by a single step but by the nature of the intermediate carbocation.
Misconception 3: The presence of chiral centers automatically leads to stereospecific addition.
Reality: While chiral centers can influence the stereochemical outcome, they don't guarantee stereospecific syn or anti addition in acid-catalyzed hydration. The key factor is the chirality (or lack thereof) of the carbocation intermediate.
Alternative Methods for Syn and Anti Addition to Alkenes
If syn or anti addition to alkenes is the desired outcome, alternative methods must be employed. These include:
-
Oxymercuration-demercuration: This reaction proceeds via a cyclic mercurinium ion intermediate, resulting in anti addition.
-
Hydroboration-oxidation: This reaction involves the addition of borane (BH₃) followed by oxidation, leading to syn addition.
Conclusion: A Comprehensive Understanding
Acid-catalyzed alkene hydration, while seemingly simple, offers a rich learning opportunity regarding regio- and stereoselectivity. Understanding the mechanism, particularly the planar nature of the carbocation intermediate, is crucial for predicting the outcome. While generally not stereospecific, leading to racemic mixtures, exceptions exist with chiral carbocations. It is essential to distinguish this reaction from other alkene addition reactions that specifically exhibit syn or anti selectivity, such as oxymercuration-demercuration and hydroboration-oxidation. Clear understanding of these nuances allows for the accurate prediction and control of product stereochemistry in organic synthesis. Remember that while Markovnikov's rule governs regioselectivity, the stereochemical outcome largely depends on the nature of the carbocation intermediate, clarifying the frequent misconception about syn or anti stereospecificity in this reaction.
Latest Posts
Latest Posts
-
What Is The Magnification Of Microscope
Mar 30, 2025
-
Testing For Cations And Anions Report Sheet
Mar 30, 2025
-
Periodic Table Elements With Protons Neutrons And Electrons
Mar 30, 2025
-
Lewis Dot Diagram For Ionic Compounds
Mar 30, 2025
-
What Is The Basic Building Block Of Nucleic Acids
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Is Acid Catalyzed Hydration Syn Or Anti . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
