Periodic Table Elements With Protons Neutrons And Electrons
Muz Play
Mar 30, 2025 · 6 min read
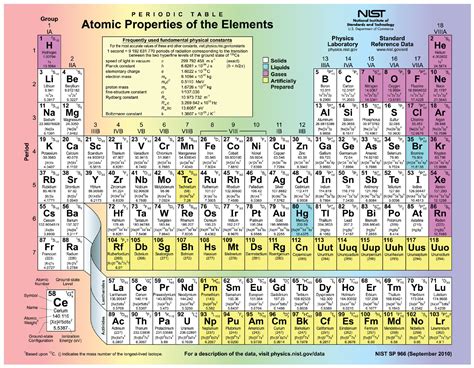
Table of Contents
Understanding the Periodic Table: A Deep Dive into Protons, Neutrons, and Electrons
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding this organization requires a grasp of the fundamental subatomic particles: protons, neutrons, and electrons. This article provides a comprehensive overview of these particles, their roles in defining elements, and their impact on the periodic table's arrangement.
The Building Blocks of Matter: Protons, Neutrons, and Electrons
Atoms, the basic units of matter, are composed of three primary subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines an element's atomic number and its identity. For example, all hydrogen atoms have one proton, all helium atoms have two, and so on. The proton's mass is approximately 1 atomic mass unit (amu).
-
Neutrons: Electrically neutral particles also found within the atom's nucleus. Unlike protons, the number of neutrons in an atom can vary, leading to isotopes of the same element. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. Like protons, neutrons have a mass of approximately 1 amu.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. Electrons are significantly lighter than protons and neutrons, with a mass approximately 1/1836 of an amu. The number of electrons in a neutral atom is equal to the number of protons. The arrangement of electrons in these shells determines an element's chemical properties and how it interacts with other elements.
Atomic Number and Mass Number: Key Identifiers of Elements
Two crucial numbers characterize each element on the periodic table:
-
Atomic Number (Z): This represents the number of protons in an atom's nucleus. It uniquely identifies an element. For instance, hydrogen (H) has an atomic number of 1, helium (He) has an atomic number of 2, and so on. The atomic number is always a whole number.
-
Mass Number (A): This is the total number of protons and neutrons in an atom's nucleus. It's approximately equal to the atom's atomic mass. The mass number is also a whole number, but unlike the atomic number, it can vary for isotopes of the same element. For example, Carbon-12 has a mass number of 12 (6 protons + 6 neutrons), while Carbon-14 has a mass number of 14 (6 protons + 8 neutrons).
Isotopes: Variations within an Element
Isotopes are atoms of the same element that have the same atomic number (number of protons) but different mass numbers (number of neutrons). Many elements exist as a mixture of isotopes. While isotopes have the same chemical properties due to their identical number of electrons, their physical properties might slightly differ due to the varying mass. Some isotopes are stable, while others are radioactive, meaning they undergo spontaneous decay, emitting particles or energy. Examples of isotopes include Carbon-12 and Carbon-14, both with six protons but with different numbers of neutrons (6 and 8, respectively).
Electron Shells and Energy Levels: Dictating Chemical Behavior
Electrons occupy specific energy levels or shells around the nucleus. These shells are designated by integers (n = 1, 2, 3, etc.), with the shell closest to the nucleus having the lowest energy. Each shell can hold a maximum number of electrons:
- Shell 1 (n=1): Holds a maximum of 2 electrons
- Shell 2 (n=2): Holds a maximum of 8 electrons
- Shell 3 (n=3): Holds a maximum of 18 electrons
- And so on...
The outermost shell, called the valence shell, contains the valence electrons. These electrons participate in chemical bonding, determining an element's reactivity and how it forms compounds with other elements. Elements with full valence shells are generally unreactive (noble gases).
The Periodic Table's Organization: A Reflection of Atomic Structure
The periodic table's arrangement reflects the periodic recurrence of similar chemical properties as atomic number increases. Elements are organized into periods (rows) and groups (columns):
-
Periods: Elements in the same period have the same number of electron shells. As you move across a period, the number of protons and electrons increases, resulting in changes in chemical properties.
-
Groups: Elements in the same group have the same number of valence electrons, leading to similar chemical behaviors. For instance, Group 1 (alkali metals) all have one valence electron, making them highly reactive. Group 18 (noble gases) have full valence shells, explaining their inert nature.
Understanding Trends Across the Periodic Table
The periodic table reveals important trends in properties as you move across periods and down groups:
-
Atomic Radius: Generally increases down a group (due to added electron shells) and decreases across a period (due to increased nuclear charge pulling electrons closer).
-
Ionization Energy: The energy required to remove an electron. Generally decreases down a group (electrons are further from the nucleus) and increases across a period (increased nuclear attraction).
-
Electronegativity: The ability of an atom to attract electrons in a chemical bond. Generally decreases down a group and increases across a period.
-
Metallic Character: The tendency of an element to lose electrons and form positive ions. Generally increases down a group and decreases across a period.
Exploring Specific Element Groups and Their Properties
Let's delve into some key groups in the periodic table:
Alkali Metals (Group 1):
Highly reactive metals with one valence electron. They readily lose this electron to form +1 ions. Examples include lithium (Li), sodium (Na), and potassium (K).
Alkaline Earth Metals (Group 2):
Reactive metals with two valence electrons. They readily lose these electrons to form +2 ions. Examples include beryllium (Be), magnesium (Mg), and calcium (Ca).
Halogens (Group 17):
Highly reactive nonmetals with seven valence electrons. They readily gain one electron to form -1 ions. Examples include fluorine (F), chlorine (Cl), and bromine (Br).
Noble Gases (Group 18):
Inert gases with full valence shells. They rarely form compounds due to their stable electronic configuration. Examples include helium (He), neon (Ne), and argon (Ar).
The Importance of Understanding Protons, Neutrons, and Electrons
A thorough understanding of protons, neutrons, and electrons is crucial for comprehending:
- Chemical bonding: How atoms interact to form molecules and compounds.
- Chemical reactions: The processes by which substances transform into new substances.
- Nuclear reactions: Processes involving changes in an atom's nucleus, such as radioactive decay.
- Material science: The properties and behavior of materials, from metals and polymers to semiconductors.
- Nuclear medicine: The use of radioactive isotopes for medical diagnosis and treatment.
Conclusion: A Journey into the Heart of Matter
The periodic table, with its organization based on the number of protons, neutrons, and electrons, provides a powerful framework for understanding the properties and behaviors of elements. By grasping the fundamental concepts of atomic structure and the trends in the periodic table, we can unlock a deeper understanding of the world around us—from the smallest atoms to the most complex molecules and materials. Further exploration into specific elements and their applications within various fields allows for even greater insights into the remarkable diversity and interconnectedness of matter. Continued study and research in this field continue to expand our knowledge of the universe and its fundamental building blocks.
Latest Posts
Latest Posts
-
Is A Base A Proton Acceptor
Apr 01, 2025
-
Area Of A Parallelogram Cross Product
Apr 01, 2025
-
Completa Estas Oraciones Con Las Preposiciones Por O Para
Apr 01, 2025
-
Cartilage Is Separated From Surrounding Tissues By A Fibrous
Apr 01, 2025
-
Archaea And Bacteria Are Most Similar In Terms Of Their
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table Elements With Protons Neutrons And Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
