Is Br- A Good Leaving Group
Muz Play
Mar 25, 2025 · 5 min read
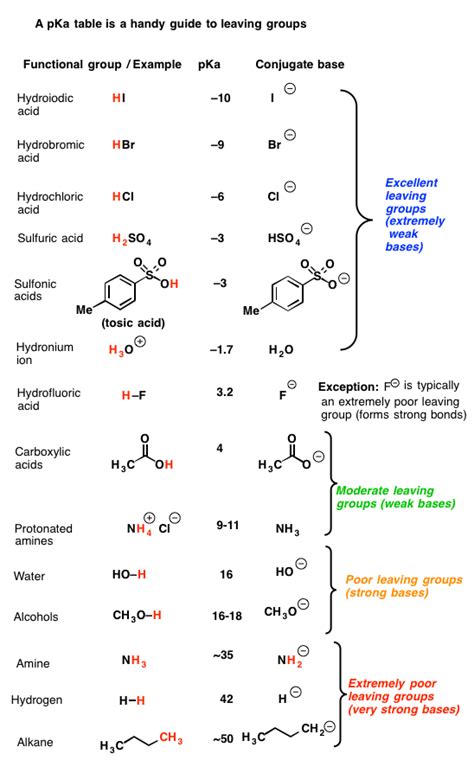
Table of Contents
Is Br- a Good Leaving Group? A Comprehensive Analysis
The question of whether bromide (Br⁻) is a good leaving group is a fundamental concept in organic chemistry. Understanding leaving group ability is crucial for predicting the reactivity and outcome of numerous reactions, including SN1, SN2, E1, and E2 reactions. While the simple answer is "yes," a deeper dive reveals nuances and factors that influence its effectiveness. This article will comprehensively explore the characteristics of Br⁻ as a leaving group, comparing it to other common leaving groups and examining the conditions under which it excels or may be less effective.
Understanding Leaving Groups
A leaving group (LG) is an atom or group of atoms that departs from a molecule, taking with it a pair of electrons. Good leaving groups are those that readily accept a pair of electrons, stabilizing the negative charge that results from their departure. Several factors contribute to a group's ability to act as a good leaving group:
Key Factors Determining Leaving Group Ability
-
Stability of the Conjugate Acid: The stronger the conjugate acid of the leaving group, the better the leaving group. This means that groups that form stable anions are better leaving groups. The stability is influenced by factors like resonance, inductive effects, and atom size.
-
Electronegativity: More electronegative atoms can better handle the negative charge after leaving. This is why halogens are generally good leaving groups.
-
Size: Larger atoms can better accommodate the negative charge due to a lower charge density. This is a significant factor favoring Br⁻ over smaller halogens like Cl⁻ or F⁻.
-
Resonance Stabilization: If the leaving group can be stabilized through resonance, it will be a better leaving group. For example, carboxylate ions are excellent leaving groups due to resonance stabilization.
Br⁻: A Detailed Examination
Bromide ion (Br⁻) is considered a good leaving group due to several favorable characteristics:
-
Strong Conjugate Acid: The conjugate acid of Br⁻ is hydrobromic acid (HBr), a strong acid. This signifies the stability of the bromide ion after it departs.
-
High Electronegativity: Bromine is highly electronegative, meaning it can effectively stabilize the negative charge it carries after leaving the molecule.
-
Large Size: Compared to other halogens like chlorine and fluorine, bromine's larger size helps distribute the negative charge, reducing its density and enhancing stability.
-
Polarizability: Bromine's larger size also contributes to its greater polarizability, enabling better interaction with the solvent and further stabilizing the anion.
Comparing Br⁻ to Other Leaving Groups
Let's compare Br⁻ to other common leaving groups to illustrate its position in the hierarchy of leaving group ability:
| Leaving Group | Conjugate Acid Strength | Electronegativity | Size | Stability | Overall Leaving Group Ability |
|---|---|---|---|---|---|
| I⁻ (iodide) | Strong | High | Largest | Highest | Excellent |
| Br⁻ (bromide) | Strong | High | Large | High | Good |
| Cl⁻ (chloride) | Strong | High | Medium | Moderate | Fair |
| F⁻ (fluoride) | Weak | High | Smallest | Lowest | Poor |
| H₂O (water) | Weak | Moderate | Small | Low | Poor |
| NH₃ (ammonia) | Weak | Moderate | Small | Low | Very Poor |
| OH⁻ (hydroxide) | Weak | High | Small | Low | Very Poor |
| -OR (alkoxide) | Weak | Moderate | Small | Low | Poor |
As the table indicates, Br⁻ sits comfortably as a good leaving group, outperforming weaker leaving groups like hydroxide (OH⁻), water (H₂O), and alkoxides (-OR). However, it is slightly less effective than iodide (I⁻), which is considered an excellent leaving group due to its even larger size and greater polarizability.
Factors Influencing Br⁻'s Effectiveness
While Br⁻ is generally a good leaving group, several factors can influence its effectiveness in specific reactions:
-
Solvent Effects: Polar protic solvents, like water or alcohols, can better stabilize the developing negative charge on the leaving group, facilitating its departure. Aprotic solvents, on the other hand, can hinder the process.
-
Substrate Structure: Steric hindrance around the carbon atom bearing the Br⁻ can affect the rate of reaction. Bulky groups can impede the approach of a nucleophile, reducing the reaction rate, particularly in SN2 reactions.
-
Nucleophile Strength: A strong nucleophile can more readily displace the leaving group.
-
Reaction Conditions: Temperature and concentration also play a role in the reaction rate.
Br⁻ in Specific Reaction Types
Br⁻'s role as a leaving group is crucial in various reaction mechanisms:
SN1 Reactions
In SN1 reactions (substitution nucleophilic unimolecular), the leaving group departs first, creating a carbocation intermediate. Br⁻, being a good leaving group, readily departs, allowing the carbocation to form. The stability of the carbocation is a key factor determining the rate of SN1 reactions; more substituted carbocations are more stable.
SN2 Reactions
In SN2 reactions (substitution nucleophilic bimolecular), the nucleophile attacks the carbon atom simultaneously with the departure of the leaving group. While Br⁻ is a good leaving group, the reaction rate is also influenced by steric factors. Bulky groups around the carbon atom can hinder the nucleophilic attack, slowing down the reaction.
Elimination Reactions (E1 and E2)
Br⁻ also plays a vital role in elimination reactions. In E1 reactions (elimination unimolecular), the leaving group departs first, forming a carbocation, which is then deprotonated to form a double bond. In E2 reactions (elimination bimolecular), the base abstracts a proton while the leaving group departs simultaneously. Br⁻'s good leaving group ability facilitates both E1 and E2 reactions.
Conclusion: Br⁻'s Position in the Leaving Group Hierarchy
In summary, bromide ion (Br⁻) is undeniably a good leaving group. Its strong conjugate acid, high electronegativity, large size, and polarizability contribute to its ability to readily depart from a molecule, facilitating various reaction mechanisms. While not the best leaving group (iodide holds that title), Br⁻'s effectiveness makes it a prevalent and valuable component in countless organic chemistry reactions. Understanding the factors influencing its effectiveness, such as solvent effects, substrate structure, and reaction conditions, is key to predicting and controlling reaction outcomes. Its reliability and versatility make it a cornerstone in the arsenal of organic chemists. The specific reaction type and conditions will ultimately dictate the success of Br⁻ as a leaving group, highlighting the dynamic nature of organic reactions and the importance of considering multiple factors for accurate predictions.
Latest Posts
Latest Posts
-
What Is The Product Of The Hydrogenation Of An Alkene
Mar 26, 2025
-
Social Contract And The Declaration Of Independence
Mar 26, 2025
-
Is Mrna Processing Is Same For Prokaryote And Eukaryote
Mar 26, 2025
-
Magnetic Field For A Bar Magnet
Mar 26, 2025
-
What Is A Common Property Of Metals
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Is Br- A Good Leaving Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
