Is Burning A Chemical Or Physical Change
Muz Play
Mar 21, 2025 · 5 min read
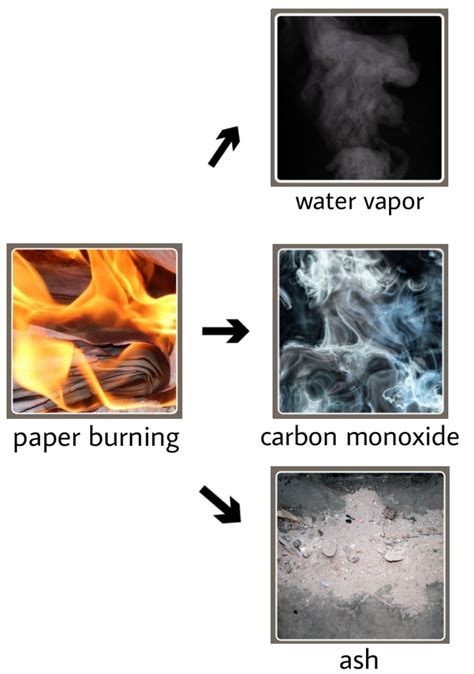
Table of Contents
Is Burning a Chemical or Physical Change? A Deep Dive into Combustion
Burning, or combustion, is a fundamental process we encounter daily, from lighting a match to powering a car. But is it a chemical change or a physical change? The answer, definitively, is chemical. This article delves deep into the reasons why, exploring the intricacies of chemical reactions, the characteristics of combustion, and the evidence that solidifies its classification as a chemical change.
Understanding Chemical vs. Physical Changes
Before we tackle the burning question (pun intended!), let's clarify the difference between chemical and physical changes.
Physical Changes: A Matter of Form, Not Substance
Physical changes alter the form or appearance of a substance but do not change its chemical composition. Think of cutting paper, melting ice, or dissolving sugar in water. The paper is still paper, the water is still water, and the sugar is still sugar—just in a different state or dispersed within another substance. These changes are reversible in many cases. You can refreeze water, or, although more difficult, reconstitute the cut paper in theory. Key indicators of a physical change include:
- Change in state: Solid to liquid, liquid to gas, etc.
- Change in shape or size: Cutting, bending, breaking.
- Change in texture: Crushing, grinding.
- No new substance is formed.
Chemical Changes: A Transformation at the Molecular Level
Chemical changes, also known as chemical reactions, involve the rearrangement of atoms and molecules to form new substances with different properties. These changes are often irreversible. Signs that a chemical change has occurred include:
- Formation of a gas: Bubbles, fizzing.
- Formation of a precipitate: A solid forms from a solution.
- Color change: A significant and permanent change in color.
- Temperature change: Exothermic reactions release heat (get hotter), while endothermic reactions absorb heat (get colder).
- Light emission: Production of light.
- A new substance with different properties is formed.
The Irrefutable Evidence: Why Burning is a Chemical Change
Burning, or combustion, ticks all the boxes for a chemical change. Let's examine the evidence:
1. Formation of New Substances: The Products of Combustion
When a substance burns, it reacts with an oxidant, typically oxygen, to produce entirely new substances. The original substance is fundamentally transformed. For example, consider the combustion of methane (natural gas):
CH₄ (methane) + 2O₂ (oxygen) → CO₂ (carbon dioxide) + 2H₂O (water)
This equation clearly shows that methane and oxygen react to create completely different substances: carbon dioxide and water. These new substances have vastly different physical and chemical properties from the original reactants. Methane is a flammable gas, while carbon dioxide is a non-flammable gas, and water is a liquid at room temperature. This transformation is a hallmark of a chemical change.
2. Release of Energy: Exothermic Reactions
Combustion is a highly exothermic reaction, meaning it releases a significant amount of energy in the form of heat and often light. This energy release is a strong indicator of a chemical change, as it signifies the breaking and forming of chemical bonds—a process that involves a substantial energy transfer. The heat generated during burning can be harnessed for various applications, from cooking to generating electricity.
3. Irreversibility: You Can't Unburn Something
Once a substance has undergone combustion, it's virtually impossible to reverse the process and recover the original substance in its initial form. The chemical bonds have been broken and reformed, creating new molecular structures. You can't magically turn the carbon dioxide and water produced by burning methane back into methane and oxygen. This irreversibility is a key characteristic of chemical changes.
4. Observable Changes: Color, Smell, and State
Burning often involves dramatic and easily observable changes. The color of the burning substance might change, smoke (a mixture of gases and particles) might be produced, and a characteristic smell often accompanies the process. These observable changes further support the conclusion that a chemical reaction has occurred. The appearance of ashes, a residue left after combustion, is another physical manifestation of the chemical changes that have occurred.
Different Types of Combustion & Their Chemical Changes
Combustion isn't a monolithic process; it encompasses various types, each involving specific chemical reactions:
Complete Combustion: Sufficient Oxygen
Complete combustion occurs when there's an ample supply of oxygen. The fuel reacts completely with oxygen, producing primarily carbon dioxide and water. This type of combustion is generally cleaner, producing fewer pollutants. However, even complete combustion results in the formation of entirely new chemical species, therefore confirming its chemical nature.
Incomplete Combustion: Limited Oxygen
Incomplete combustion happens when the oxygen supply is limited. This results in the production of carbon monoxide (CO), a toxic gas, and soot (unburned carbon particles) in addition to carbon dioxide and water. The formation of these additional byproducts further underscores the chemical transformation occurring during burning. The different products highlight the impact of varying reaction conditions on the outcome of a chemical reaction.
Spontaneous Combustion: Heat Buildup
Spontaneous combustion is a special case where a substance ignites without an external ignition source. This usually occurs due to the slow oxidation of a material, generating heat that accumulates until it reaches the ignition point. The underlying process, even if seemingly gradual, still involves chemical reactions and the formation of new substances.
The Role of Activation Energy
Combustion requires an activation energy—the minimum energy needed to initiate the reaction. This energy can be supplied through various means, like a spark, a flame, or friction. Once this activation energy is provided, the exothermic reaction proceeds spontaneously, releasing even more energy. This energy barrier is another key aspect of the chemical nature of burning; physical changes do not typically require such an energy input to proceed.
Conclusion: Burning as a Defining Chemical Process
The evidence overwhelmingly demonstrates that burning, or combustion, is a chemical change, not a physical change. The formation of new substances with different properties, the release of energy, the irreversibility of the process, and the observable changes all point to a fundamental transformation at the molecular level. Understanding the chemical nature of combustion is crucial in various fields, from fire safety to energy production and environmental science. By recognizing that burning is a chemical process, we can better understand its implications and develop strategies to control and utilize this powerful phenomenon. The profound transformation at the molecular level during combustion sets it apart definitively from physical alterations.
Latest Posts
Latest Posts
-
On The Weak Acid Strong Base Titration Curve
Mar 28, 2025
-
Label The Cranial Dura Septa In The Figure
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is Burning A Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
