Is Combustion Of Gasoline A Chemical Change
Muz Play
Mar 18, 2025 · 6 min read
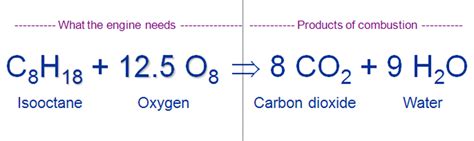
Table of Contents
Is the Combustion of Gasoline a Chemical Change? A Deep Dive
The roar of a car engine, the whoosh of a jet plane taking off – these are all testaments to the power of combustion, a process often simplified as burning. But is the combustion of gasoline truly a chemical change? The answer, unequivocally, is yes. This article will delve into the intricacies of gasoline combustion, exploring the chemical reactions, observable changes, and the scientific principles that solidify its classification as a chemical change. We'll also examine some common misconceptions and explore the broader implications of this fundamental chemical process.
Understanding Chemical Changes
Before diving into the specifics of gasoline combustion, let's establish a clear understanding of what constitutes a chemical change. Unlike a physical change, which alters the form or appearance of a substance without changing its chemical composition (e.g., melting ice), a chemical change involves the rearrangement of atoms to form new substances with different properties. Key indicators of a chemical change include:
- Formation of a new substance: This is the most crucial indicator. The products have distinct properties from the reactants.
- Evolution of gas: The release of gases is a common sign of a chemical reaction.
- Change in color: A noticeable shift in color often indicates a chemical transformation.
- Formation of a precipitate: The appearance of a solid from a solution is another strong indicator.
- Change in temperature: Exothermic reactions release heat, while endothermic reactions absorb heat.
The Chemical Composition of Gasoline
Gasoline is a complex mixture of hydrocarbons, primarily alkanes, with a carbon chain length typically ranging from 4 to 12 carbon atoms. These hydrocarbons are volatile organic compounds (VOCs) that readily evaporate at room temperature. The exact composition of gasoline can vary depending on the refinery and the season (summer blends generally have a higher volatility). However, the core components remain hydrocarbons, which are the primary players in the combustion process.
Key Components and their roles:
- Alkanes (e.g., octane, heptane): These form the backbone of gasoline and are the main fuel source during combustion.
- Additives: Various additives are included to enhance performance and meet environmental regulations. These include detergents, anti-knock agents (like MTBE), and oxygenates. While these additives play a role in the overall combustion process, the fundamental chemical change remains the reaction of the hydrocarbons.
The Combustion Reaction: A Detailed Look
The combustion of gasoline is essentially a rapid oxidation reaction, where the hydrocarbons in gasoline react with oxygen (O2) from the air. This reaction is highly exothermic, meaning it releases a significant amount of energy in the form of heat and light. The complete combustion of a simple alkane, such as octane (C8H18), can be represented by the following balanced chemical equation:
2C₈H₁₈ (l) + 25O₂ (g) → 16CO₂ (g) + 18H₂O (g) + Energy
This equation shows that octane reacts with oxygen to produce carbon dioxide (CO2), water (H2O), and energy. The (l), (g) notations indicate the physical state of the reactants and products – liquid, and gas, respectively.
Incomplete Combustion: A Less Efficient Process
In reality, complete combustion is rarely achieved in an internal combustion engine. Incomplete combustion occurs when there isn't enough oxygen available for the complete oxidation of the hydrocarbons. This results in the formation of byproducts like carbon monoxide (CO), unburnt hydrocarbons, and particulate matter (soot). Incomplete combustion is less efficient and releases less energy than complete combustion. The equation for incomplete combustion can vary depending on the specific byproducts formed. For example, one possibility involving carbon monoxide is:
2C₈H₁₈ (l) + 17O₂ (g) → 16CO (g) + 18H₂O (g) + Energy
The formation of CO, a highly toxic gas, highlights the importance of ensuring sufficient oxygen supply during combustion for efficient and safe operation.
Observable Changes Confirming Chemical Change
Several observable changes clearly demonstrate that gasoline combustion is a chemical change:
- Energy Release (Heat and Light): The significant release of heat and light is a hallmark of an exothermic chemical reaction. The heat produced drives the pistons in a car engine, while the light is evident in the flames of a fire.
- Formation of New Substances: The combustion products, CO2 and H2O, are distinctly different from the reactants, gasoline and oxygen. Their properties – such as their boiling points, reactivity, and toxicity – differ significantly.
- Evolution of Gases: The production of carbon dioxide and water vapor as gases is a clear indicator of a chemical reaction. These gases are expelled through the exhaust system of a vehicle.
- Change in Color (Flame): The characteristic color of the flame produced during gasoline combustion (ranging from yellow to blue depending on the completeness of combustion) further confirms a chemical transformation.
Addressing Common Misconceptions
Several misconceptions surrounding gasoline combustion need clarification:
- "Burning is just a physical change": Burning is a chemical process involving the breaking and reforming of chemical bonds, resulting in the formation of entirely new substances. This fundamental alteration of the molecular structure confirms that it's not simply a change in physical state.
- "Only explosions are chemical changes": While explosions are dramatic chemical changes, many other chemical reactions, including the relatively slower combustion of gasoline in an engine, also represent chemical changes. The speed of the reaction doesn't determine its classification.
Environmental Implications and Mitigation
The combustion of gasoline is a significant contributor to air pollution. The release of greenhouse gases like carbon dioxide contributes to global warming, while pollutants like nitrogen oxides and particulate matter impact air quality and human health. However, advancements in engine technology and fuel formulations have led to substantial reductions in emissions. The development of hybrid and electric vehicles further aims to mitigate the environmental impact of gasoline combustion.
Strategies for cleaner combustion:
- Catalytic Converters: These devices reduce harmful emissions like nitrogen oxides and unburnt hydrocarbons.
- Improved Engine Design: Optimizing engine design to promote more complete combustion can minimize the formation of harmful byproducts.
- Fuel Additives: Certain additives can enhance combustion efficiency and reduce emissions.
- Alternative Fuels: Exploring and implementing alternative fuels like biofuels and hydrogen can offer more sustainable alternatives to gasoline.
Conclusion: A Definitive Chemical Transformation
In conclusion, the combustion of gasoline is undeniably a chemical change. The evidence—from the rearrangement of atoms to the formation of new substances and the release of energy—irrefutably supports this classification. Understanding the chemical processes involved is crucial, not only for optimizing engine performance and efficiency but also for mitigating the environmental impact of this fundamental energy source. The continued research and development in cleaner combustion technologies will play a pivotal role in shaping a more sustainable future for transportation and energy production. The journey from simple observation of a flame to a deep understanding of the underlying chemical reactions highlights the power and importance of scientific inquiry.
Latest Posts
Latest Posts
-
Which Type Of Hormone Is Lipid Soluble
Mar 18, 2025
-
How To Find Instantaneous Rate Of Reaction
Mar 18, 2025
-
Meiosis I And Meiosis Ii Different
Mar 18, 2025
-
How Many Protons Are In Sulfur
Mar 18, 2025
-
Organic Chemistry 1 Final Exam Review
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Combustion Of Gasoline A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
