Is Mass Number Protons Plus Neutrons
Muz Play
May 10, 2025 · 6 min read
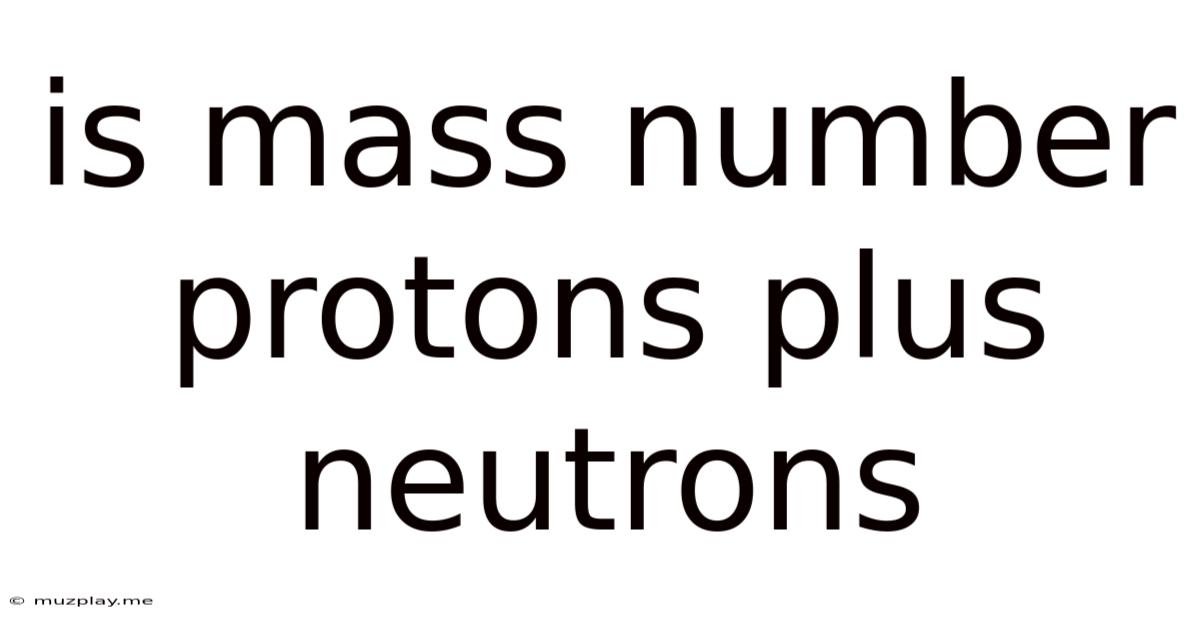
Table of Contents
Is Mass Number Protons Plus Neutrons? A Deep Dive into Atomic Structure
The simple answer is yes, the mass number of an atom is the sum of its protons and neutrons. This fundamental concept underpins our understanding of atomic structure and is crucial in various fields, from chemistry and physics to nuclear medicine and materials science. However, a deeper exploration reveals nuances and complexities that enrich our understanding beyond this basic definition. This article will delve into the intricacies of atomic mass, isotopes, and the implications of this seemingly straightforward equation.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before diving into the mass number, let's refresh our understanding of the basic building blocks of an atom. Atoms are composed of three primary subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element; for example, all atoms with one proton are hydrogen, those with two are helium, and so on. This number is known as the atomic number.
-
Neutrons: Neutrally charged particles also found in the nucleus. Unlike protons, the number of neutrons can vary within the same element, leading to the existence of isotopes.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons usually equals the number of protons in a neutral atom, ensuring a balanced charge.
Mass Number: The Sum of Protons and Neutrons
The mass number (A), as the name suggests, represents the total mass of an atom. Since the mass of electrons is negligible compared to protons and neutrons, the mass number is essentially the sum of the number of protons (Z, the atomic number) and the number of neutrons (N):
A = Z + N
For instance, a carbon-12 atom has 6 protons and 6 neutrons. Therefore, its mass number is 12 (6 + 6 = 12). This is often represented as ¹²C, where the superscript denotes the mass number.
Isotopes: Variations in Neutron Number
The beauty of this equation lies in its ability to explain the existence of isotopes. Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. This difference in neutron number results in variations in the atom's mass number, but not in its chemical properties.
Consider the example of carbon:
- ¹²C (Carbon-12): 6 protons, 6 neutrons, mass number 12. This is the most abundant isotope of carbon.
- ¹³C (Carbon-13): 6 protons, 7 neutrons, mass number 13. This is a stable isotope, though less abundant than ¹²C.
- ¹⁴C (Carbon-14): 6 protons, 8 neutrons, mass number 14. This is a radioactive isotope used in carbon dating.
Notice that all three are carbon atoms because they all have 6 protons. However, their mass numbers differ due to the varying number of neutrons. This highlights the importance of considering both the atomic number and the mass number for a complete atomic description.
Atomic Mass: The Weighted Average of Isotopes
The mass number provides the mass of a specific isotope. However, elements in nature usually exist as a mixture of isotopes. Therefore, we use the atomic mass (or atomic weight), which is the weighted average of the masses of all the naturally occurring isotopes of an element. The weighting is based on the relative abundance of each isotope.
For example, while the mass number of ¹²C is 12 and that of ¹³C is 13, the atomic mass of carbon is approximately 12.011 amu (atomic mass units). This slight deviation from 12 is due to the presence of a small amount of ¹³C in naturally occurring carbon.
Implications of Mass Number: Beyond Simple Addition
While the mass number being the sum of protons and neutrons is a foundational concept, it's crucial to understand that it's not a perfectly precise representation of an atom's mass. Several factors contribute to this:
-
Mass Defect: The mass of a nucleus is slightly less than the sum of the masses of its individual protons and neutrons. This difference, known as the mass defect, is due to the strong nuclear force binding the nucleons (protons and neutrons) together. This energy released in binding is described by Einstein's famous equation, E=mc².
-
Binding Energy: The energy required to separate the nucleons in a nucleus is called the binding energy. This energy is directly related to the mass defect and is a crucial factor in nuclear stability and reactions.
-
Isotopic Abundance: The precise atomic mass of an element reflects the weighted average of its isotopes' masses and their natural abundances. Variations in isotopic ratios can occur depending on the source of the element.
Applications of Mass Number and Isotopes
Understanding mass numbers and isotopes is crucial in various scientific and technological applications:
-
Nuclear Medicine: Radioactive isotopes are used in diagnostic imaging techniques like PET (positron emission tomography) and SPECT (single-photon emission computed tomography) to visualize internal organs and detect diseases. The choice of isotope is based on its specific decay properties and mass number.
-
Radiocarbon Dating: ¹⁴C, with its known half-life, is used to date organic materials, allowing us to determine the age of artifacts and fossils. The ratio of ¹⁴C to ¹²C provides crucial information.
-
Nuclear Power: Nuclear reactors utilize isotopes like Uranium-235 for nuclear fission, releasing energy for electricity generation. The mass number is crucial in determining the fissile nature of the isotope.
-
Material Science: Understanding the isotopic composition of materials can influence their physical and chemical properties. This is significant in fields like semiconductor production and materials characterization.
-
Analytical Chemistry: Mass spectrometry techniques utilize the mass-to-charge ratio of ions to analyze the isotopic composition of samples. This allows for precise determination of atomic masses and isotopic abundances.
Conclusion: A Foundation for Deeper Understanding
While the simple statement "mass number equals protons plus neutrons" is a fundamental truth in atomic structure, exploring the nuances surrounding it unlocks a deeper appreciation for the intricacies of atomic physics and chemistry. Understanding isotopes, atomic mass, mass defect, and binding energy reveals the complexity hidden within this seemingly straightforward equation. This knowledge forms the bedrock for numerous advancements in diverse fields, emphasizing the profound significance of this basic yet powerful concept. The mass number, therefore, is not just a simple sum; it's a key to unlocking the mysteries of the atom and its behavior. Furthermore, appreciating the subtleties related to mass number, isotopic abundance and atomic mass showcases the elegance and precision of the scientific method in uncovering the fundamental building blocks of our universe. This detailed understanding allows us to harness the power of atoms for various technological applications and advancements in various scientific fields. Further exploration into nuclear physics and chemistry is highly encouraged for those seeking a more in-depth understanding of this fascinating topic.
Latest Posts
Latest Posts
-
Research On Sex Hormones And Animal Sexual Behavior Indicates That
May 10, 2025
-
Choose The Quadratic Model For The Situation
May 10, 2025
-
Complete And Balance The Following Precipitation Reactions
May 10, 2025
-
What Is The Magnifying Power Of The Low Power Objective
May 10, 2025
-
According To The Logistic Growth Equation
May 10, 2025
Related Post
Thank you for visiting our website which covers about Is Mass Number Protons Plus Neutrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.