Is Salt A Homogeneous Or Heterogeneous Mixture
Muz Play
Mar 22, 2025 · 5 min read
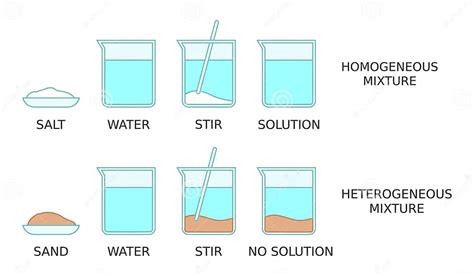
Table of Contents
- Is Salt A Homogeneous Or Heterogeneous Mixture
- Table of Contents
- Is Salt a Homogeneous or Heterogeneous Mixture? A Deep Dive into Matter
- Understanding Homogeneous and Heterogeneous Mixtures
- The Case of Salt: A Closer Look
- 1. Dry Salt Crystals:
- 2. Salt Dissolved in Water:
- 3. Saltwater with Undissolved Salt:
- Why the Distinction Matters: Applications and Implications
- Other Types of Mixtures and Classifications
- Conclusion: Salt's Homogeneity (mostly)
- Latest Posts
- Latest Posts
- Related Post
Is Salt a Homogeneous or Heterogeneous Mixture? A Deep Dive into Matter
The question of whether salt is a homogeneous or heterogeneous mixture often arises in chemistry and science education. Understanding the difference between these two types of mixtures is crucial for grasping fundamental concepts of matter and its properties. This article will delve into the nature of salt, exploring its chemical composition, physical properties, and ultimately definitively answering whether it's homogeneous or heterogeneous. We'll also explore related concepts to solidify your understanding.
Understanding Homogeneous and Heterogeneous Mixtures
Before we tackle the salt question, let's clearly define our terms:
Homogeneous Mixture: A homogeneous mixture is a type of mixture where the components are uniformly distributed throughout the mixture. This means that the composition is consistent throughout and you won't be able to visually distinguish the individual components. Think of saltwater – the salt dissolves completely, creating a uniform solution. Other examples include air (a mixture of gases), sugar dissolved in water, and many alloys. The key is uniformity at the macroscopic level.
Heterogeneous Mixture: In contrast, a heterogeneous mixture has components that are not uniformly distributed. You can visually identify the different components. Examples include sand and water, oil and water, and a salad. The composition varies from one part of the mixture to another.
The Case of Salt: A Closer Look
Salt, in its common culinary form, is primarily sodium chloride (NaCl). When we talk about table salt, we're often referring to a refined form of sodium chloride, but it may also contain small amounts of additives like iodine or anticaking agents.
Let's consider salt in different scenarios:
1. Dry Salt Crystals:
If you look at a pile of dry table salt crystals, you might initially think it's heterogeneous because you can see individual crystals of varying sizes. However, this is a deceptive observation. While the crystals themselves are distinct, the chemical composition of each crystal is essentially identical – NaCl. The size difference doesn't alter the chemical makeup. At the level of individual crystals, it's homogeneous. At a larger scale of a pile of crystals, it may appear heterogeneous due to the size variation of the crystals, but the chemical composition of each individual crystal remains the same. Therefore, even dry salt crystals can be considered homogeneous at the chemical level.
2. Salt Dissolved in Water:
When salt dissolves in water, it forms a solution. The sodium chloride (NaCl) molecules dissociate into sodium (Na+) and chloride (Cl-) ions, which are evenly dispersed throughout the water molecules. You cannot visually distinguish the salt from the water. This is a classic example of a homogeneous mixture. The composition is the same regardless of which part of the solution you sample. The microscopic distribution of ions and water molecules is uniform.
3. Saltwater with Undissolved Salt:
If you add more salt to water than can dissolve (creating a saturated solution and leaving undissolved crystals at the bottom), then you have a heterogeneous mixture. There are two distinct phases: the saturated saltwater solution (homogeneous) and the solid, undissolved salt crystals (homogeneous). The overall mixture, however, is heterogeneous because of the presence of these two distinct phases.
Why the Distinction Matters: Applications and Implications
The distinction between homogeneous and heterogeneous mixtures is important in various fields:
-
Chemistry: Understanding the type of mixture helps determine the appropriate separation techniques. For example, filtration is suitable for separating components of a heterogeneous mixture, whereas distillation or chromatography might be used for homogeneous mixtures.
-
Material Science: The properties of materials often depend on whether they are homogeneous or heterogeneous. The uniform distribution of components in a homogeneous alloy, for example, contributes to its strength and other desirable properties.
-
Environmental Science: Understanding the distribution of pollutants in air or water (homogeneous or heterogeneous) is vital for effective environmental monitoring and remediation.
-
Food Science: The texture and properties of food products are often influenced by whether their components form homogeneous or heterogeneous mixtures.
-
Pharmaceutical Science: The effectiveness and bioavailability of drugs can depend on whether they are formulated as homogeneous or heterogeneous mixtures. For example, a uniform distribution of a drug within a dosage form ensures consistent delivery.
Other Types of Mixtures and Classifications
While homogeneous and heterogeneous mixtures are the most common classifications, it's worth briefly mentioning other types of mixtures:
-
Suspensions: These are heterogeneous mixtures where particles are dispersed in a liquid, but they settle over time if left undisturbed (e.g., muddy water).
-
Colloids: These are mixtures where particles are dispersed in a medium, but they are smaller than those in a suspension and don't settle out (e.g., milk).
Conclusion: Salt's Homogeneity (mostly)
In summary, pure salt (sodium chloride) is inherently homogeneous at a molecular level. Whether a mixture containing salt is homogeneous or heterogeneous depends on the context. A solution of salt dissolved in water is a homogeneous mixture. A pile of dry salt crystals is practically homogeneous at a macroscopic level due to the identical composition of its individual crystals. However, a mixture of salt and water with undissolved salt present is a heterogeneous mixture. Understanding these distinctions is key to appreciating the intricate nature of matter and the behavior of mixtures in various scientific and everyday contexts. The key takeaway is to analyze the mixture at the appropriate scale and consider whether the composition is uniform throughout.
Latest Posts
Latest Posts
-
Who Wrote The Magic Flute Opera
Mar 23, 2025
-
Convert From Cylindrical To Spherical Coordinates
Mar 23, 2025
-
Parts Of The Compound Light Microscope
Mar 23, 2025
-
How To Flush Right In Latex Mathjax
Mar 23, 2025
-
Hydrogen Bonds Can Be Broken By
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Is Salt A Homogeneous Or Heterogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
