Is Temp And Pressure Directly Proportional
Muz Play
Mar 15, 2025 · 5 min read
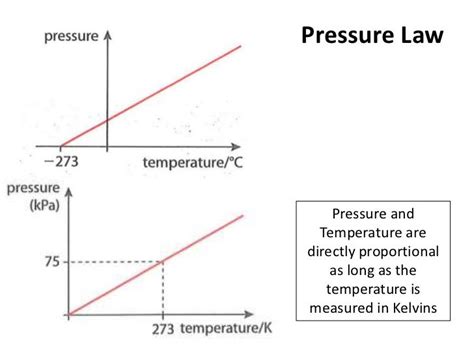
Table of Contents
Is Temperature and Pressure Directly Proportional? Exploring the Relationship in Ideal Gases and Real-World Scenarios
The relationship between temperature and pressure is a fundamental concept in physics and chemistry, particularly when studying gases. A common misconception is that temperature and pressure are always directly proportional. While this holds true under specific, idealized conditions, the reality is far more nuanced. This article delves into the intricacies of this relationship, exploring the ideal gas law, the limitations of its applicability, and the factors that influence the temperature-pressure connection in real-world scenarios.
Understanding the Ideal Gas Law: The Foundation of Temperature-Pressure Relationship
The ideal gas law serves as the cornerstone for understanding the relationship between temperature and pressure in gases. It's expressed mathematically as:
PV = nRT
Where:
- P represents pressure
- V represents volume
- n represents the number of moles of gas
- R is the ideal gas constant
- T represents temperature (in Kelvin)
If we keep the volume (V) and the number of moles (n) constant, the equation simplifies to:
P/T = constant or P₁/T₁ = P₂/T₂
This simplified form shows a direct proportionality between pressure and temperature: as temperature increases, pressure increases proportionally, and vice versa. This holds true only for ideal gases under constant volume.
Ideal Gas Assumptions: The Fine Print
It's crucial to remember that the ideal gas law rests on several assumptions that don't always reflect reality:
- Negligible intermolecular forces: Ideal gases are assumed to have no attractive or repulsive forces between gas particles. In reality, these forces exist and affect the pressure exerted by the gas.
- Particles with negligible volume: The volume of individual gas particles is considered negligible compared to the volume of the container. This assumption breaks down at high pressures where the particle volume becomes significant.
- Elastic collisions: Collisions between gas particles and the container walls are assumed to be perfectly elastic, meaning no kinetic energy is lost during collisions. Real-world collisions involve some energy loss.
Beyond the Ideal: Real Gases and Their Behavior
Real gases deviate from the ideal gas law, especially at high pressures and low temperatures. Under these conditions, the intermolecular forces and the finite volume of gas molecules become significant, affecting the pressure-temperature relationship.
The Influence of Intermolecular Forces
Attractive forces between gas molecules cause them to stick together slightly, reducing the number of collisions with the container walls and thus lowering the pressure. This effect becomes more pronounced at lower temperatures where the kinetic energy of the molecules is reduced, allowing the attractive forces to dominate.
The Role of Molecular Volume
At high pressures, the volume occupied by the gas molecules themselves becomes a significant fraction of the total volume of the container. This reduces the available space for the molecules to move around, leading to a higher pressure than predicted by the ideal gas law.
Compressibility Factor: Measuring Deviations from Ideality
The compressibility factor (Z) is a useful parameter to quantify the deviation of a real gas from ideal behavior. It's defined as:
Z = PV/nRT
For an ideal gas, Z = 1. For real gases, Z can be greater than or less than 1, depending on the conditions and the nature of the gas. A Z value greater than 1 indicates a higher pressure than expected for an ideal gas (due to the volume of the molecules), while a Z value less than 1 indicates a lower pressure (due to intermolecular attractions).
Real-World Scenarios: Where Direct Proportionality Breaks Down
While the ideal gas law provides a valuable approximation, understanding its limitations is essential when analyzing real-world scenarios. Here are a few examples:
High-Pressure Systems: Compressed Gases
In systems with high pressure, such as compressed gas cylinders, the volume of the gas molecules and intermolecular forces significantly affect the pressure-temperature relationship. The direct proportionality observed in ideal gases no longer holds true. An increase in temperature will lead to a pressure increase, but the magnitude of this increase will be different from what the ideal gas law predicts. The compressibility factor will be significantly greater than 1.
Low-Temperature Systems: Liquefaction of Gases
At low temperatures, intermolecular attractive forces become dominant. As temperature decreases, these forces cause the gas molecules to clump together, leading to condensation and the formation of a liquid. The pressure-temperature relationship becomes highly complex and non-linear in this phase transition region.
Reactions Involving Gases: Chemical Kinetics
When chemical reactions involving gases occur, the number of moles of gas can change. This directly impacts the pressure exerted by the gas mixture, even if the temperature remains constant. Therefore, simple direct proportionality between temperature and pressure doesn't apply in such scenarios. The ideal gas law needs to be applied carefully, considering changes in 'n' (number of moles).
Modifying the Ideal Gas Law for Real Gases: Equations of State
To improve the accuracy of the ideal gas law for real gases, several equations of state have been developed. These equations incorporate terms that account for intermolecular forces and molecular volume. Examples include the van der Waals equation, the Redlich-Kwong equation, and the Peng-Robinson equation. These equations provide more accurate predictions of pressure-temperature relationships under various conditions, particularly when dealing with non-ideal gases.
Conclusion: A Complex and Nuanced Relationship
The relationship between temperature and pressure in gases is not always a simple direct proportionality. While the ideal gas law provides a useful framework for understanding this relationship under specific conditions, it's crucial to remember its limitations. Real gases deviate from ideal behavior, especially at high pressures and low temperatures, where intermolecular forces and molecular volume play significant roles. To accurately describe the temperature-pressure relationship in real-world scenarios, a more sophisticated approach, using modified equations of state and careful consideration of system conditions, is often necessary. The direct proportionality described by the simplified ideal gas law serves as a useful starting point but should not be blindly applied without acknowledging the complexities of real-world gas behavior. Understanding these nuances is vital for accurately modeling and predicting gas behavior in various applications, ranging from industrial processes to atmospheric science.
Latest Posts
Latest Posts
-
What Elemsnts Are Most Likey To Turn Into Anions Why
Mar 15, 2025
-
What Is The Difference Between Hunger And Appetite
Mar 15, 2025
-
Boiling Point On Graph In Celsius
Mar 15, 2025
-
List The Classification Levels From Broadest To Most Specific
Mar 15, 2025
-
Equipments For Measuring Volume Of Acids
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is Temp And Pressure Directly Proportional . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
