Is The Conjugate Base Of A Weak Acid Strong
Muz Play
Mar 15, 2025 · 6 min read
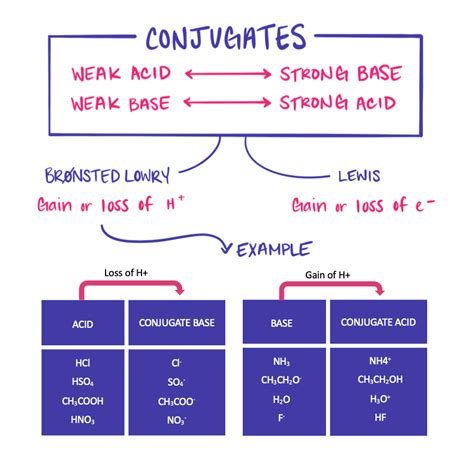
Table of Contents
Is the Conjugate Base of a Weak Acid Strong? Understanding Acid-Base Conjugate Pairs
The relationship between acids and bases is a cornerstone of chemistry, and understanding conjugate acid-base pairs is crucial for grasping many chemical concepts. A common question that arises is: is the conjugate base of a weak acid strong? The short answer is no, but the nuance behind this answer requires a deeper exploration of acid-base chemistry and the principles that govern their behavior. This article delves into the specifics of weak acids, their conjugate bases, and the factors influencing their strengths and weaknesses.
Understanding Weak Acids and Their Dissociation
A weak acid is a substance that only partially dissociates in water, meaning it doesn't completely break apart into its constituent ions (H⁺ and its conjugate base). Instead, it establishes an equilibrium between the undissociated acid (HA) and its ions (H⁺ and A⁻). This equilibrium is represented by the following equation:
HA(aq) ⇌ H⁺(aq) + A⁻(aq)
The equilibrium constant for this reaction, denoted as K<sub>a</sub>, is the acid dissociation constant. A smaller K<sub>a</sub> value indicates a weaker acid, signifying a lower tendency to donate protons (H⁺). The lower the K<sub>a</sub>, the less it dissociates, and therefore, the weaker the acid.
Examples of Weak Acids
Numerous common substances qualify as weak acids. Examples include:
- Acetic acid (CH₃COOH): Found in vinegar.
- Formic acid (HCOOH): Present in ant stings.
- Carbonic acid (H₂CO₃): Formed when carbon dioxide dissolves in water, crucial in blood buffering.
- Hydrocyanic acid (HCN): A highly toxic weak acid.
- Benzoic acid (C₇H₆O₂): Used as a food preservative.
Conjugate Acid-Base Pairs: A Definition
According to the Brønsted-Lowry theory of acids and bases, an acid is a proton (H⁺) donor, while a base is a proton acceptor. When an acid donates a proton, it forms its conjugate base, which is the species remaining after the proton is released. Conversely, when a base accepts a proton, it forms its conjugate acid. The conjugate acid-base pair differs by only a single proton.
For example, in the dissociation of acetic acid:
CH₃COOH(aq) ⇌ H⁺(aq) + CH₃COO⁻(aq)
- CH₃COOH is the acid.
- CH₃COO⁻ is its conjugate base.
The Strength of Conjugate Bases
The strength of a conjugate base is inversely related to the strength of its parent acid. This means:
- The conjugate base of a strong acid is weak. Strong acids completely dissociate, leaving behind a conjugate base that has a very low affinity for protons.
- The conjugate base of a weak acid is weak, but not as weak as the conjugate base of a strong acid. Weak acids only partially dissociate, meaning their conjugate bases still possess some affinity for protons, albeit less than the parent acid.
Why the Conjugate Base of a Weak Acid Isn't Strong
The conjugate base of a weak acid still has some ability to accept a proton and reform the weak acid. This is because the equilibrium established during the weak acid's dissociation lies significantly to the left (favoring the undissociated acid). The conjugate base, therefore, does not readily react with water to produce hydroxide ions (OH⁻) – a characteristic of strong bases.
Quantifying Base Strength: K<sub>b</sub>
The strength of a base is quantified by its base dissociation constant, K<sub>b</sub>. K<sub>b</sub> represents the equilibrium constant for the reaction of a base with water:
A⁻(aq) + H₂O(l) ⇌ HA(aq) + OH⁻(aq)
A larger K<sub>b</sub> indicates a stronger base. For conjugate acid-base pairs, the relationship between K<sub>a</sub> and K<sub>b</sub> is given by:
K<sub>a</sub> × K<sub>b</sub> = K<sub>w</sub>
where K<sub>w</sub> is the ion product constant for water (approximately 1.0 × 10⁻¹⁴ at 25°C). This equation highlights the inverse relationship between the strength of an acid and its conjugate base.
Factors Affecting the Strength of Conjugate Bases
Several factors influence the strength of a conjugate base, including:
- Electronegativity: A more electronegative atom in the conjugate base can better stabilize the negative charge, making it a weaker base.
- Size of the anion: Larger anions can better distribute the negative charge, leading to weaker basicity.
- Resonance stabilization: If the conjugate base can exhibit resonance, the negative charge is delocalized, resulting in a weaker base.
- Inductive effects: Electron-withdrawing groups can stabilize the negative charge on the conjugate base, making it less basic.
Examples Illustrating Conjugate Base Strength
Let's examine some specific examples to solidify our understanding:
-
Acetic acid (CH₃COOH) and its conjugate base (CH₃COO⁻): Acetic acid is a weak acid, and its conjugate base, acetate ion, is also a weak base. Acetate ions can accept protons but do not readily react with water to produce a significant concentration of hydroxide ions.
-
Hydrochloric acid (HCl) and its conjugate base (Cl⁻): Hydrochloric acid is a strong acid, and its conjugate base, chloride ion, is an extremely weak base. The chloride ion has virtually no tendency to accept protons.
-
Ammonia (NH₃) and its conjugate acid (NH₄⁺): Ammonia is a weak base, and its conjugate acid, ammonium ion, is a weak acid.
The Importance of Understanding Conjugate Acid-Base Pairs
Understanding the relationship between acids, bases, and their conjugates is vital for various applications, including:
- Buffer solutions: Buffer solutions are essential in maintaining a stable pH in biological systems and chemical processes. They typically consist of a weak acid and its conjugate base or a weak base and its conjugate acid.
- Titrations: Acid-base titrations rely on the neutralization reaction between an acid and a base, involving conjugate acid-base pairs.
- Understanding biochemical processes: Many biological molecules act as weak acids or bases, and their conjugate pairs play critical roles in enzyme activity and other cellular processes.
Conclusion: Weak Acid, Weak Conjugate Base
In conclusion, the conjugate base of a weak acid is not strong. While it possesses some basicity, its ability to accept protons is significantly less than that of strong bases. This behavior stems from the equilibrium established during the weak acid's dissociation and is governed by the acid dissociation constant (K<sub>a</sub>) and the base dissociation constant (K<sub>b</sub>). The relative strength of the conjugate base depends on factors like electronegativity, size, resonance, and inductive effects within the conjugate base structure. A thorough grasp of these principles is essential for comprehending acid-base chemistry and its numerous applications across various scientific disciplines.
Latest Posts
Latest Posts
-
What Elemsnts Are Most Likey To Turn Into Anions Why
Mar 15, 2025
-
What Is The Difference Between Hunger And Appetite
Mar 15, 2025
-
Boiling Point On Graph In Celsius
Mar 15, 2025
-
List The Classification Levels From Broadest To Most Specific
Mar 15, 2025
-
Equipments For Measuring Volume Of Acids
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is The Conjugate Base Of A Weak Acid Strong . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
