Is Water An Ionic Or Covalent Compound
Muz Play
Mar 23, 2025 · 6 min read
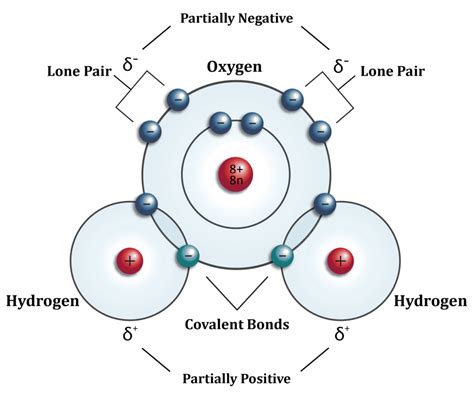
Table of Contents
Is Water an Ionic or Covalent Compound? Delving into the Nature of Water's Bonds
Water, the elixir of life, is a seemingly simple molecule with a deceptively complex nature. Its unique properties, crucial for sustaining life as we know it, stem directly from the type of chemical bond holding its atoms together. The question, "Is water an ionic or covalent compound?", is a fundamental one in chemistry, and understanding the answer unlocks a deeper appreciation for water's remarkable characteristics. The short answer is: water is a covalent compound. But let's delve into the details to truly grasp why.
Understanding Chemical Bonds: Ionic vs. Covalent
Before we classify water, let's clarify the differences between ionic and covalent bonds. These are the two primary types of chemical bonds that hold atoms together to form molecules and compounds.
Ionic Bonds: The Electrostatic Attraction
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. This happens when one atom donates an electron (or electrons) to another atom. The atom that loses electrons becomes a positively charged ion (cation), while the atom that gains electrons becomes a negatively charged ion (anion). The strong attraction between these opposite charges forms the ionic bond. A classic example is sodium chloride (NaCl), or table salt, where sodium (Na) loses an electron to chlorine (Cl), forming Na+ and Cl- ions, respectively. Ionic compounds generally have high melting and boiling points due to the strong electrostatic forces holding them together. They also tend to be soluble in water and conduct electricity when dissolved or molten.
Covalent Bonds: Sharing is Caring
Covalent bonds, on the other hand, are formed by the sharing of electrons between atoms. This sharing occurs when atoms achieve a more stable electron configuration by sharing electrons in their outermost shell (valence shell). Instead of transferring electrons completely like in ionic bonds, atoms in covalent bonds share electrons to reach a full valence shell. The shared electrons are attracted to the nuclei of both atoms, creating a bond. Covalent compounds generally have lower melting and boiling points than ionic compounds because the covalent bonds are weaker than ionic bonds. They are often insoluble in water and do not conduct electricity.
The Covalent Nature of Water: A Detailed Look
Water (H₂O) is formed by two hydrogen atoms and one oxygen atom. Oxygen is significantly more electronegative than hydrogen. Electronegativity refers to an atom's ability to attract electrons in a chemical bond. Oxygen's higher electronegativity means it attracts the shared electrons more strongly than hydrogen. However, the difference in electronegativity isn't large enough to cause a complete transfer of electrons, as would happen in an ionic bond. Instead, the electrons are shared unequally, resulting in a polar covalent bond.
Polar Covalent Bonds: Unequal Sharing
In a polar covalent bond, the shared electrons spend more time closer to the more electronegative atom, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the less electronegative atom. In water, the oxygen atom carries a partial negative charge (δ-), while the hydrogen atoms carry partial positive charges (δ+). This unequal distribution of charge creates a dipole moment, making the water molecule polar.
The Significance of Water's Polarity
The polar nature of water is critical to its unique properties. This polarity allows water molecules to form hydrogen bonds with each other and with other polar molecules. Hydrogen bonds are relatively weak compared to covalent or ionic bonds, but they are significant in influencing water's behavior. These bonds are responsible for many of water's exceptional properties:
- High boiling point: Hydrogen bonds require considerable energy to break, resulting in a relatively high boiling point for water compared to other molecules of similar size.
- High specific heat capacity: Water can absorb a significant amount of heat without a large temperature change due to the energy required to break hydrogen bonds. This property is crucial for regulating temperature in living organisms and the environment.
- High surface tension: Hydrogen bonds create a strong cohesive force between water molecules, resulting in high surface tension.
- Excellent solvent: Water's polarity allows it to dissolve many ionic and polar substances, making it an excellent solvent for biological processes.
- Density anomaly: Ice is less dense than liquid water because the hydrogen bonds in ice create a more open, crystalline structure. This anomaly is essential for aquatic life, as ice floats, insulating the water beneath.
Debunking Misconceptions: Why Water Isn't Ionic
While some might mistakenly consider water ionic because it can dissolve ionic compounds, this is a misunderstanding. Water's ability to dissolve ionic compounds stems from its polarity, not its ionic nature. The partial positive charges on the hydrogen atoms attract the anions, and the partial negative charge on the oxygen atom attracts the cations, effectively separating the ions and dissolving the compound. The water molecules surround the ions, preventing them from recombining. This process of solvation is facilitated by water's dipole moment, a direct consequence of its polar covalent bonds.
Further Exploring the Properties of Water
Water's unique properties, derived from its covalent nature and the resulting polarity and hydrogen bonding, are fundamental to life's existence. Let's briefly explore some key properties in more detail:
1. Cohesion and Adhesion
Cohesion, the attraction between water molecules, and adhesion, the attraction between water and other polar substances, are crucial for processes like capillary action in plants, where water is drawn up against gravity.
2. Universal Solvent
Water's ability to dissolve a wide range of substances is essential for biological processes, allowing for the transport of nutrients and the removal of waste products. This solvency is a direct consequence of its polar nature and ability to form hydrogen bonds.
3. Temperature Regulation
Water's high specific heat capacity and high heat of vaporization help regulate temperature fluctuations, protecting organisms from drastic temperature changes. This capacity is vital for maintaining stable internal temperatures in living beings.
4. Density Anomaly of Ice
The lower density of ice compared to liquid water prevents bodies of water from freezing solid, allowing aquatic life to survive in winter conditions. This unique property ensures that ecosystems can maintain life throughout the colder months.
Conclusion: Water – A Covalent Masterpiece
In conclusion, the unequivocal answer is that water is a covalent compound, specifically a polar covalent compound. Its unique properties, so vital to life on Earth, are a direct result of the unequal sharing of electrons between oxygen and hydrogen atoms, leading to a polar molecule capable of forming hydrogen bonds. Understanding the nature of water's bonds is essential for grasping its remarkable and life-sustaining properties. The apparent simplicity of the H₂O formula belies the intricate interplay of forces that make water the extraordinary substance it is. The study of water's chemical nature is a continuing journey of discovery, revealing ever more about this fundamental molecule's profound impact on our world.
Latest Posts
Latest Posts
-
Which Kingdoms Contain Organisms That Are Prokaryotes
Mar 25, 2025
-
What Element Is Found In Proteins
Mar 25, 2025
-
2 Sample Z Test For Proportions
Mar 25, 2025
-
Last Of Five Rhyming Greek Letters
Mar 25, 2025
-
Rusting Of Iron Is Chemical Or Physical Change
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Is Water An Ionic Or Covalent Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
