Is Work Done On The System Positive
Muz Play
Apr 01, 2025 · 6 min read
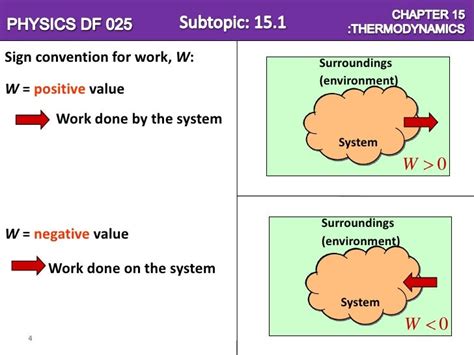
Table of Contents
Is Work Done on the System Positive? A Comprehensive Exploration of Thermodynamics
Determining whether work done on a system is positive or negative hinges on a crucial understanding of thermodynamic conventions and the perspective from which the work is viewed. This article delves deep into this fundamental concept, exploring various scenarios, providing clear examples, and clarifying common misconceptions. We'll unpack the intricacies of work, energy transfer, and the system-surrounding interactions that define these thermodynamic processes.
Understanding the System and Surroundings
Before diving into the positivity or negativity of work, let's establish a clear definition of the system and its surroundings. In thermodynamics, a system is the specific part of the universe we are interested in studying, while the surroundings encompass everything else. The boundary separates the system from its surroundings.
Consider a gas contained within a piston cylinder. The gas itself is the system, while the piston, cylinder, and the surrounding air represent the surroundings. Work done on or by the system always involves energy transfer across this boundary.
The Sign Convention: Work Done On the System
The key to understanding the sign convention lies in the perspective: work done on the system is considered positive (+). This means that the system is gaining energy from its surroundings. Conversely, work done by the system is negative (-), indicating that the system is losing energy to its surroundings.
This seemingly simple convention is crucial in accurately tracking energy changes within a thermodynamic process. The convention is based on the change in the system's internal energy (ΔU). If the system gains energy, ΔU is positive, and this energy gain is often a result of positive work done on the system.
Examples of Positive Work Done on the System
Several scenarios exemplify positive work done on the system:
-
Compressing a gas: Imagine pushing down on the piston of a cylinder containing a gas. You are doing work on the gas, increasing its internal energy and pressure. This work is positive (+W). The gas's volume decreases.
-
Lifting a weight: If you lift a weight, you are doing positive work on the weight. The weight gains potential energy, a form of internal energy.
-
Charging a battery: When you charge a battery, external energy is transferred to the battery, thereby performing positive work on the system (the battery). The battery's internal energy increases.
-
Stretching a spring: Stretching a spring requires applying a force over a distance, thereby performing positive work on the spring. This increases the spring's potential energy.
The Sign Convention: Work Done By the System
Conversely, work done by the system is considered negative (-). This indicates that the system is losing energy to its surroundings. This energy loss often manifests as mechanical work, such as expansion against external pressure.
Examples of Negative Work Done by the System
Examples of negative work done by the system include:
-
Expanding a gas: If the gas in a piston cylinder expands, it does work on the surroundings (pushing the piston outward), and therefore work done by the system is negative (-W). The gas loses energy.
-
Dropping a weight: When a weight drops, it does work on its surroundings (e.g., accelerating other objects). The work done by the system (the weight) is negative.
-
Discharging a battery: As a battery discharges, it does work on the external circuit, and the work done by the system (the battery) is negative.
-
Releasing a compressed spring: When a compressed spring is released, it performs work on its surroundings as it returns to its equilibrium state. The work done by the system (the spring) is negative.
The First Law of Thermodynamics and Work
The first law of thermodynamics, also known as the law of conservation of energy, provides the framework for understanding these sign conventions:
ΔU = Q + W
Where:
- ΔU is the change in the system's internal energy.
- Q is the heat transferred to the system. A positive Q indicates heat absorbed by the system, while a negative Q means heat is released by the system.
- W is the work done on the system. A positive W indicates work done on the system, while a negative W indicates work done by the system.
This equation underscores the importance of correctly assigning signs to both Q and W to accurately account for all energy transfers.
Work and Different Thermodynamic Processes
The nature of the work done also depends on the type of thermodynamic process:
Isobaric Processes (Constant Pressure)
In isobaric processes, the pressure remains constant. The work done is calculated as:
W = -PΔV
Where:
- P is the constant pressure
- ΔV is the change in volume
Notice the negative sign. If the volume increases (ΔV > 0), the work done is negative (work done by the system). If the volume decreases (ΔV < 0), the work done is positive (work done on the system).
Isochoric Processes (Constant Volume)
In isochoric processes, the volume remains constant. Since no change in volume occurs (ΔV = 0), no work is done (W = 0).
Isothermal Processes (Constant Temperature)
In isothermal processes, the temperature remains constant. The work done is calculated using calculus and depends on the equation of state of the system.
Adiabatic Processes (No Heat Exchange)
In adiabatic processes, no heat is exchanged with the surroundings (Q = 0). The change in internal energy is solely determined by the work done: ΔU = W. Therefore, a positive ΔU implies positive work done on the system, and a negative ΔU implies negative work done by the system.
Common Misconceptions
Several misunderstandings frequently arise regarding the sign convention for work:
-
Confusing force and work: The direction of the force applied does not directly determine the sign of the work. The sign depends on whether the system gains or loses energy due to the work.
-
Ignoring the system's perspective: The crucial aspect is the perspective—whether the work increases or decreases the system's internal energy.
-
Misinterpreting expansion and compression: Expansion often implies negative work (by the system), while compression often implies positive work (on the system), but this is not an absolute rule.
Practical Applications and Significance
Understanding the sign convention for work is paramount in many fields:
-
Engineering: Designing engines, turbines, and other mechanical systems requires precise calculations of work done.
-
Chemistry: Studying chemical reactions and their energy changes necessitates accurate accounting of work done.
-
Physics: Analyzing thermodynamic processes, especially in areas like statistical mechanics, relies heavily on this understanding.
-
Environmental Science: Assessing energy efficiency and environmental impact requires careful consideration of work done in various systems.
Conclusion
The sign convention for work in thermodynamics, positive for work done on the system and negative for work done by the system, is fundamental to understanding energy transfers and applying the first law of thermodynamics. By consistently applying this convention, we can accurately model and analyze a wide range of thermodynamic processes, leading to accurate predictions and better engineering design. Remembering that the perspective is always from the system helps clarify this crucial aspect of thermodynamics. This comprehensive understanding is not merely an academic exercise; it underpins many practical applications across various scientific and engineering disciplines. Mastering this concept is key to mastering thermodynamics itself.
Latest Posts
Latest Posts
-
Thesis Statement For Narrative Essay Example
Apr 02, 2025
-
Inborn Nonspecific Defenses Include And Barriers
Apr 02, 2025
-
Jewish Murals From The First Century Ce Depict
Apr 02, 2025
-
Which Quadratic Function Is Represented By The Graph
Apr 02, 2025
-
An Organized Arrangement Of Elements According To Their Atomic Number
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Work Done On The System Positive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
