Isotopes Differ In The Number Of
Muz Play
Mar 23, 2025 · 6 min read
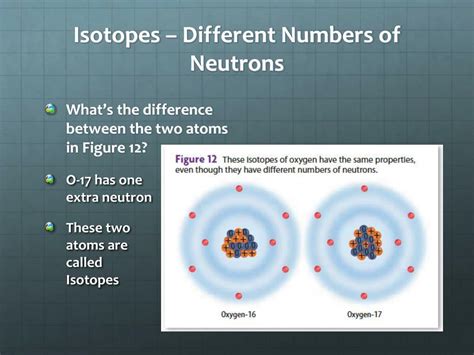
Table of Contents
Isotopes Differ in the Number of Neutrons: A Deep Dive into Atomic Structure and Isotopic Variations
Isotopes are variations of a chemical element that possess the same number of protons but differ in the number of neutrons within their atomic nuclei. This seemingly subtle difference has profound implications across various scientific fields, impacting everything from nuclear medicine to geological dating. Understanding isotopic variations requires a foundational grasp of atomic structure and the forces that govern nuclear stability. This comprehensive article delves into the intricacies of isotopes, exploring their properties, applications, and the significance of their neutron count.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we dive into isotopes, let's establish a solid understanding of the basic building blocks of an atom. Every atom consists of three primary subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the atomic number of an element and determines its identity on the periodic table. For example, all hydrogen atoms have one proton, all carbon atoms have six, and so on.
-
Neutrons: Neutral particles (no charge) also found within the nucleus. Unlike protons, the number of neutrons in an atom can vary without changing the element's identity. This variation leads to the existence of isotopes.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons typically equals the number of protons in a neutral atom, maintaining an overall neutral charge. However, atoms can gain or lose electrons, forming ions with a net positive or negative charge.
The mass of an atom is primarily determined by the number of protons and neutrons in its nucleus, as electrons have significantly less mass. This mass is expressed in atomic mass units (amu), where one amu is approximately the mass of a single proton or neutron.
What are Isotopes? The Defining Difference: Neutron Number
Isotopes are atoms of the same element that share the same number of protons but differ in their number of neutrons. This difference in neutron number leads to variations in the atom's mass but doesn't alter its chemical properties significantly. The chemical properties of an element are primarily determined by the number of electrons and their arrangement in the electron shells, which is directly influenced by the number of protons.
Example: Carbon Isotopes
Carbon (atomic number 6) has three naturally occurring isotopes:
- Carbon-12 (¹²C): Contains 6 protons and 6 neutrons. This is the most abundant isotope of carbon.
- Carbon-13 (¹³C): Contains 6 protons and 7 neutrons. This is a stable isotope, though less abundant than ¹²C.
- Carbon-14 (¹⁴C): Contains 6 protons and 8 neutrons. This is a radioactive isotope, meaning its nucleus is unstable and decays over time. This radioactive decay is utilized in radiocarbon dating.
The number after the element's name represents the mass number (A), which is the sum of protons and neutrons in the nucleus (A = Z + N, where Z is the atomic number and N is the neutron number). Thus, all isotopes of a given element have the same atomic number (Z) but different mass numbers (A).
Isotopic Notation and Representation
Isotopes are typically represented using isotopic notation:
A
X
Z
Where:
- X is the element's chemical symbol (e.g., C for carbon, U for uranium).
- Z is the atomic number (number of protons).
- A is the mass number (number of protons + neutrons).
For example, Carbon-14 is represented as ¹⁴₆C.
Nuclear Stability and Radioactive Isotopes
The stability of an atomic nucleus depends on the balance between the strong nuclear force (which holds protons and neutrons together) and the electromagnetic force (which causes protons to repel each other). Isotopes with a particular ratio of protons to neutrons are more stable than others. Isotopes with an unstable nucleus are called radioactive isotopes or radioisotopes.
Radioactive isotopes undergo radioactive decay, transforming into a different element by emitting particles or energy. The rate of decay is characterized by the isotope's half-life, which is the time it takes for half of the atoms in a sample to decay. Radioactive isotopes have various applications in medicine, research, and industry, including:
-
Medical imaging: Radioisotopes like Technetium-99m are used in diagnostic imaging techniques such as single-photon emission computed tomography (SPECT).
-
Cancer treatment: Radioisotopes such as Iodine-131 and Cobalt-60 are used in radiotherapy to destroy cancer cells.
-
Radiocarbon dating: Carbon-14 dating is used to determine the age of organic materials up to around 50,000 years old.
-
Industrial tracing: Radioactive isotopes are used as tracers to follow the movement of substances in industrial processes.
Applications of Isotope Analysis: Unveiling the Secrets of Matter
The analysis of isotopic ratios in materials provides valuable insights across many disciplines:
-
Geology and Paleoclimatology: Isotopic ratios in rocks and ice cores reveal information about Earth's past climate, tectonic activity, and the formation of geological formations. For example, oxygen isotope ratios in ice cores are used to reconstruct past temperatures.
-
Forensic Science: Isotopic analysis can help trace the origin of materials, such as drugs or explosives, aiding in criminal investigations.
-
Environmental Science: Isotope ratios in water samples can track pollution sources and movement of water through ecosystems.
-
Archaeology: Isotopic analysis of bones and artifacts can provide insights into ancient diets, migration patterns, and cultural practices.
Isotopic Abundance and Atomic Weight
The atomic weight (or atomic mass) of an element listed on the periodic table is a weighted average of the masses of its naturally occurring isotopes, taking into account their relative abundances. This means that the atomic weight reflects the proportion of each isotope present in a typical sample of the element. For instance, the atomic weight of carbon is approximately 12.01 amu, which is slightly higher than 12 amu because of the presence of small amounts of ¹³C.
Isotope Separation: Techniques and Challenges
Separating isotopes from each other is a challenging process because they possess nearly identical chemical properties. Various techniques are employed for isotope separation, depending on the specific isotopes and the desired level of purity. Some common methods include:
-
Gaseous diffusion: This method exploits the slight difference in the diffusion rates of gases containing different isotopes.
-
Centrifugation: This technique utilizes centrifugal force to separate isotopes based on their mass differences.
-
Laser isotope separation: This method employs lasers to selectively excite and ionize specific isotopes, allowing for their separation.
Conclusion: The Significance of Isotopic Variations
The number of neutrons in an atom profoundly impacts its properties and applications. Isotopes, differing solely in neutron count, provide a powerful tool for scientific inquiry across diverse fields. Their study enhances our understanding of fundamental atomic structure, unveils hidden histories of the Earth and its inhabitants, and drives advancements in medicine, technology, and environmental monitoring. From radiocarbon dating to medical imaging, isotopes are integral to our modern world, demonstrating the crucial role of seemingly subtle nuclear variations in shaping scientific progress. Further research into isotopic variations promises to unlock even more secrets of the universe and further advance our understanding of the natural world.
Latest Posts
Latest Posts
-
What Is The Sliding Filament Theory Of Muscle Contraction
Mar 25, 2025
-
Calculating The Ph Of A Strong Acid Solution
Mar 25, 2025
-
A Chemical Bond Is Formed When Electrons Are
Mar 25, 2025
-
Memory That Is Active At Any Given Moment Is Called
Mar 25, 2025
-
Domain And Range In Quadratic Functions
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Isotopes Differ In The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
