Lewis Acid And Base Vs Bronsted
Muz Play
Mar 16, 2025 · 5 min read
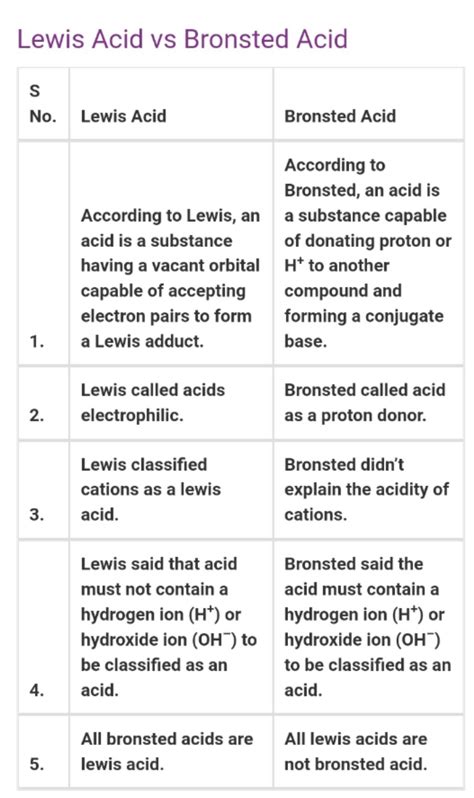
Table of Contents
Lewis Acid and Base vs. Brønsted-Lowry: A Comprehensive Comparison
Understanding acid-base chemistry is fundamental to many areas of chemistry, from organic synthesis to biochemistry. While the Brønsted-Lowry definition of acids and bases is widely taught and used, the Lewis definition provides a broader, more encompassing framework. This article delves deep into the differences and similarities between these two crucial acid-base theories, exploring their applications and limitations.
The Brønsted-Lowry Definition: Proton Transfer
The Brønsted-Lowry theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, defines acids and bases based on proton (H⁺) transfer.
- Brønsted-Lowry Acid: A species that donates a proton (H⁺) to another species.
- Brønsted-Lowry Base: A species that accepts a proton (H⁺) from another species.
This definition elegantly explains many acid-base reactions in aqueous solutions. For example, in the reaction between hydrochloric acid (HCl) and water:
HCl + H₂O ⇌ H₃O⁺ + Cl⁻
HCl acts as a Brønsted-Lowry acid, donating a proton to water, which acts as a Brønsted-Lowry base. The resulting hydronium ion (H₃O⁺) is the conjugate acid of water, and the chloride ion (Cl⁻) is the conjugate base of HCl. The concept of conjugate acid-base pairs is central to the Brønsted-Lowry theory.
Strengths and Limitations of Brønsted-Lowry Theory
The Brønsted-Lowry theory successfully explains a vast range of acid-base reactions, particularly those involving proton transfer in aqueous solutions. However, it has limitations:
- Limited Scope: It only considers reactions involving proton transfer. Many reactions that exhibit acid-base characteristics do not involve protons.
- Solvent Dependency: The theory is heavily reliant on the presence of a proton-accepting solvent, typically water. Reactions in non-protic solvents are not readily explained by this theory.
- No Explanation for Certain Reactions: Some reactions involving electron pair donation and acceptance, crucial in many organic reactions, fall outside the scope of the Brønsted-Lowry definition.
The Lewis Definition: Electron Pair Donation and Acceptance
Gilbert N. Lewis proposed a more general definition of acids and bases in 1923, focusing on electron pair interactions.
- Lewis Acid: A species that accepts an electron pair. These are often electron-deficient species, possessing vacant orbitals.
- Lewis Base: A species that donates an electron pair. These usually possess lone pairs of electrons.
This definition significantly broadens the scope of acid-base chemistry. A Lewis acid-base reaction involves the formation of a coordinate covalent bond, where both electrons in the bond originate from the Lewis base.
Examples of Lewis Acid-Base Reactions
Many reactions previously not considered acid-base reactions under the Brønsted-Lowry definition are readily explained by the Lewis definition:
- Reaction of Boron Trifluoride (BF₃) with Ammonia (NH₃): BF₃, an electron-deficient molecule with an empty p-orbital, acts as a Lewis acid. Ammonia, possessing a lone pair of electrons on the nitrogen atom, acts as a Lewis base. The reaction forms a coordinate covalent bond between boron and nitrogen:
BF₃ + :NH₃ → F₃B:NH₃
- Formation of Complex Ions: Transition metal ions, often with vacant d-orbitals, readily act as Lewis acids, forming complex ions with Lewis bases like water or ammonia. For instance, the formation of the hexaaquacopper(II) ion:
Cu²⁺ + 6H₂O → [Cu(H₂O)₆]²⁺
- Reactions of Carbonyl Compounds: The carbonyl group (C=O) in aldehydes and ketones acts as a Lewis acid, accepting electron pairs from nucleophiles (Lewis bases) in nucleophilic addition reactions.
Advantages of the Lewis Definition
The Lewis definition offers several advantages over the Brønsted-Lowry definition:
- Wider Applicability: It encompasses a much broader range of reactions, including those not involving protons.
- Explains More Reactions: It provides a unifying framework for understanding a wider variety of acid-base reactions, particularly in organic and inorganic chemistry.
- Predictive Power: It allows for better prediction of reaction outcomes based on the electron-donating and accepting capabilities of the reactants.
Limitations of the Lewis Definition
While the Lewis definition is more comprehensive, it does have some limitations:
- Less Intuitive: The concept of electron pair donation and acceptance may be less intuitive for beginners than the simple proton transfer concept.
- Broadness Can Be Overwhelming: The wide scope can make it challenging to classify some reactions definitively.
Comparing Brønsted-Lowry and Lewis Acids and Bases: A Table Summary
| Feature | Brønsted-Lowry | Lewis |
|---|---|---|
| Definition | Proton (H⁺) transfer | Electron pair donation and acceptance |
| Acid | Proton donor | Electron pair acceptor |
| Base | Proton acceptor | Electron pair donor |
| Scope | Limited to reactions involving proton transfer | Broader scope; includes reactions without protons |
| Solvent | Usually requires a protic solvent | Can occur in various solvents, including non-protic solvents |
| Examples | HCl, H₂SO₄, NH₃, H₂O | BF₃, AlCl₃, transition metal ions, carbonyl compounds |
| Conjugate Pairs | Yes | Not always directly applicable |
Applications of Both Theories
Both the Brønsted-Lowry and Lewis definitions are essential tools in understanding and predicting chemical behavior.
Brønsted-Lowry theory is heavily used in:
- Aqueous solution chemistry: Calculating pH, predicting the strength of acids and bases, and understanding buffer solutions.
- Biochemistry: Explaining the action of enzymes and the role of acids and bases in biological processes.
Lewis theory is crucial in:
- Organic chemistry: Understanding reaction mechanisms involving nucleophiles and electrophiles.
- Inorganic chemistry: Explaining the formation of coordination complexes and the behavior of transition metal ions.
- Catalysis: Many catalysts operate through Lewis acid-base interactions.
Conclusion
Both the Brønsted-Lowry and Lewis definitions of acids and bases are invaluable in chemistry. The Brønsted-Lowry definition provides a simpler, more intuitive framework for understanding proton transfer reactions, particularly in aqueous solutions. However, the Lewis definition offers a more encompassing and general perspective, explaining a much wider range of reactions based on electron pair interactions. Understanding both theories is crucial for a complete grasp of acid-base chemistry and its diverse applications across various fields of chemistry. The choice of which definition to use depends heavily on the specific chemical system and the context of the reaction being studied. Often, both theories can be used to complement each other and provide a deeper understanding of the chemical process. Ultimately, the Lewis theory can be viewed as a superset of the Brønsted-Lowry theory, encompassing the latter as a specific case within its broader framework.
Latest Posts
Latest Posts
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
-
A Temporary Mixture The Particles Will Eventually Settle
Mar 17, 2025
-
Why Do Ions Travel Back And Forth In Orbitrap
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Lewis Acid And Base Vs Bronsted . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
