Lewis Acid Vs Bronsted Lowry Acid
Muz Play
Mar 24, 2025 · 6 min read
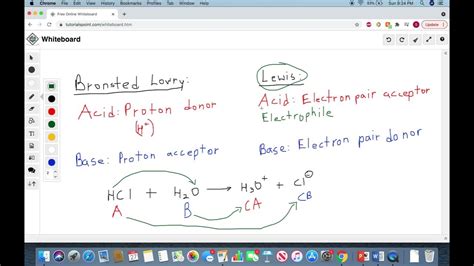
Table of Contents
- Lewis Acid Vs Bronsted Lowry Acid
- Table of Contents
- Lewis Acid vs. Brønsted-Lowry Acid: A Comprehensive Comparison
- Defining Brønsted-Lowry Acids
- Examples of Brønsted-Lowry Acids:
- Limitations of the Brønsted-Lowry Theory:
- Defining Lewis Acids
- Examples of Lewis Acids:
- Understanding Lewis Acid-Base Reactions:
- Comparing Brønsted-Lowry and Lewis Acids
- Why is every Brønsted-Lowry acid also a Lewis acid?
- Examples of Lewis Acids that are not Brønsted-Lowry Acids:
- Applications of Lewis Acid-Base Chemistry
- Practical Implications and Advanced Concepts
- Hard and Soft Acid-Base Theory (HSAB):
- Superacids:
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Lewis Acid vs. Brønsted-Lowry Acid: A Comprehensive Comparison
Understanding the concepts of acids and bases is fundamental to chemistry. While the Brønsted-Lowry definition is widely taught, the Lewis definition provides a broader perspective, encompassing reactions that don't fit neatly into the Brønsted-Lowry framework. This article delves into the differences and similarities between Lewis acids and Brønsted-Lowry acids, offering a detailed comparison for a comprehensive understanding.
Defining Brønsted-Lowry Acids
The Brønsted-Lowry theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, defines an acid as a proton (H⁺) donor and a base as a proton acceptor. This definition centers on the transfer of a proton during an acid-base reaction. A key characteristic of Brønsted-Lowry acid-base reactions is the formation of a conjugate acid-base pair. When an acid donates a proton, it forms its conjugate base, and when a base accepts a proton, it forms its conjugate acid.
Examples of Brønsted-Lowry Acids:
- Hydrochloric acid (HCl): HCl readily donates a proton to water, forming hydronium ions (H₃O⁺) and chloride ions (Cl⁻).
- Sulfuric acid (H₂SO₄): A strong diprotic acid, meaning it can donate two protons.
- Acetic acid (CH₃COOH): A weak monoprotic acid, meaning it only donates one proton. It only partially ionizes in water.
- Ammonium ion (NH₄⁺): Although not traditionally considered an acid, it acts as a Brønsted-Lowry acid by donating a proton.
Limitations of the Brønsted-Lowry Theory:
While the Brønsted-Lowry theory is highly useful, it has limitations. It fails to explain acid-base reactions that don't involve proton transfer. For example, reactions involving certain metal ions or molecules without acidic hydrogen atoms are not accounted for by this theory.
Defining Lewis Acids
Gilbert N. Lewis proposed a broader definition of acids and bases in 1923, encompassing a wider range of reactions. A Lewis acid is defined as an electron-pair acceptor, while a Lewis base is an electron-pair donor. This definition focuses on the movement of electron pairs rather than the transfer of protons.
Examples of Lewis Acids:
- Boron trifluoride (BF₃): BF₃ has an incomplete octet, making it electron-deficient. It readily accepts an electron pair from a Lewis base.
- Aluminum chloride (AlCl₃): Similar to BF₃, AlCl₃ is an electron-deficient molecule that acts as a Lewis acid. It's commonly used as a catalyst in organic chemistry.
- Transition metal ions: Many transition metal ions, particularly those with high charge densities, act as Lewis acids by accepting electron pairs from ligands. For instance, Fe³⁺ forms complexes with water molecules.
- Carbon dioxide (CO₂): While not overtly electron-deficient, the carbon atom in CO₂ can accept an electron pair from a base, such as hydroxide ion (OH⁻), leading to the formation of bicarbonate (HCO₃⁻).
- Trimethyloxonium ion [(CH₃)₃O⁺]: This ion is a Lewis acid because the positively charged oxygen atom can accept electrons.
Understanding Lewis Acid-Base Reactions:
A Lewis acid-base reaction involves the formation of a coordinate covalent bond, where both electrons in the bond are donated by the Lewis base. This contrasts with a typical covalent bond where each atom contributes one electron to the bond. The Lewis acid-base adduct is formed as a result of this electron-pair donation.
Comparing Brønsted-Lowry and Lewis Acids
The crucial difference lies in the focus: proton transfer versus electron-pair acceptance. All Brønsted-Lowry acids are also Lewis acids, but not all Lewis acids are Brønsted-Lowry acids.
| Feature | Brønsted-Lowry Acid | Lewis Acid |
|---|---|---|
| Definition | Proton (H⁺) donor | Electron-pair acceptor |
| Mechanism | Proton transfer | Electron-pair donation (coordinate bond formation) |
| Scope | Limited to reactions involving proton transfer | Broader scope; includes reactions without proton transfer |
| Examples | HCl, H₂SO₄, CH₃COOH, NH₄⁺ | BF₃, AlCl₃, Fe³⁺, CO₂, (CH₃)₃O⁺ |
| Relationship | All Brønsted-Lowry acids are Lewis acids | Not all Lewis acids are Brønsted-Lowry acids |
Why is every Brønsted-Lowry acid also a Lewis acid?
When a Brønsted-Lowry acid donates a proton (H⁺), it is essentially donating a proton that has a positive charge and no electrons. This positively charged proton can only exist if it forms a bond with an electron pair. The proton seeks out a electron pair from the Lewis base it is reacting with, making it an electron pair acceptor and thus a Lewis acid. The proton acts as an electrophile; an electron-seeking species in this sense.
Examples of Lewis Acids that are not Brønsted-Lowry Acids:
Many Lewis acids lack a proton to donate. Let's revisit some examples:
- BF₃: Boron trifluoride has no protons to donate. Its acidity stems entirely from its ability to accept an electron pair.
- AlCl₃: Aluminum chloride similarly lacks a proton to donate, acting as a Lewis acid by accepting electron pairs.
- Transition metal ions (e.g., Fe³⁺): These ions are electron-deficient and readily accept electron pairs from ligands, but they don't donate protons.
Applications of Lewis Acid-Base Chemistry
The concept of Lewis acids and bases is crucial in numerous areas of chemistry, including:
- Organic Chemistry: Lewis acids are frequently used as catalysts in various organic reactions, such as Friedel-Crafts alkylation and acylation. The Lewis acid helps to activate electrophiles to make them more reactive.
- Inorganic Chemistry: The formation of coordination complexes relies heavily on Lewis acid-base interactions, where metal ions act as Lewis acids and ligands act as Lewis bases. This is crucial in understanding the chemistry of transition metals.
- Biochemistry: Many biological processes involve Lewis acid-base interactions. For instance, enzyme catalysis often involves the interaction of a metal ion (Lewis acid) with a substrate.
- Materials Science: The synthesis and characterization of many materials involve Lewis acid-base reactions. For example, the synthesis of certain ceramics involves the interaction of metal oxides (Lewis acids) with other materials.
Practical Implications and Advanced Concepts
Understanding the distinctions between Brønsted-Lowry and Lewis acids is crucial for predicting reaction outcomes and designing effective chemical processes. The Lewis definition expands our understanding of acid-base chemistry beyond the simple transfer of protons, providing a more comprehensive framework for describing a wider range of reactions.
Hard and Soft Acid-Base Theory (HSAB):
The Hard and Soft Acid-Base (HSAB) theory, proposed by Ralph Pearson, builds on the Lewis acid-base concept. This theory categorizes Lewis acids and bases as "hard" or "soft" based on their size, charge density, and polarizability. Hard acids prefer to react with hard bases, and soft acids prefer to react with soft bases. This theory provides a more nuanced understanding of the selectivity in Lewis acid-base reactions and helps predict the stability of resulting complexes.
Superacids:
Superacids are extremely strong acids that are even stronger than 100% sulfuric acid. Many superacids are based on Lewis acid-base combinations, where a strong Lewis acid combines with a Brønsted acid to create a system with enhanced acidity. These are frequently used in advanced organic chemistry.
Conclusion
The Brønsted-Lowry and Lewis definitions of acids and bases offer distinct yet complementary perspectives on acid-base chemistry. While the Brønsted-Lowry theory focuses on proton transfer, the Lewis theory emphasizes electron-pair donation and acceptance, encompassing a broader range of reactions. A thorough understanding of both theories is essential for a complete grasp of acid-base chemistry and its applications in various fields of science. The Lewis theory, with its extensions like HSAB theory, provides a more comprehensive and predictive framework for understanding a wider range of chemical interactions. Recognizing both definitions enhances the ability to predict reaction behavior and design effective chemical strategies.
Latest Posts
Latest Posts
-
Label The Substances Involved In Facilitated Diffusion
Mar 27, 2025
-
The Heisenberg Uncertainty Principle States That
Mar 27, 2025
-
How Can Two Different Nonmetals Form A Compound
Mar 27, 2025
-
Horizontal Row On The Periodic Table
Mar 27, 2025
-
What Are 3 Properties Of Water
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Lewis Acid Vs Bronsted Lowry Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
