Lewis Dot Structure Practice Worksheet Answers
Muz Play
Mar 28, 2025 · 6 min read
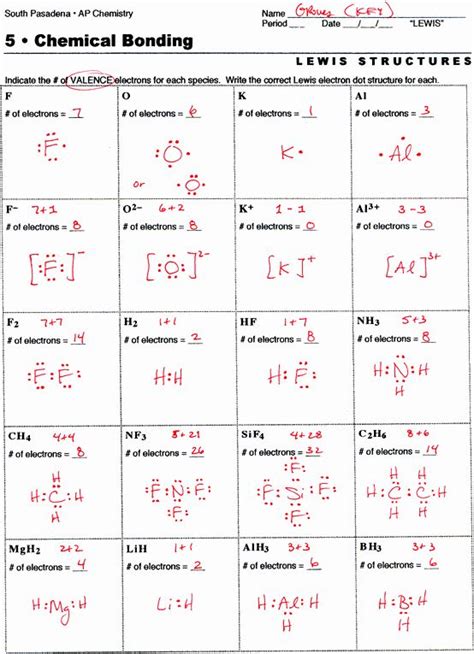
Table of Contents
Lewis Dot Structure Practice Worksheet Answers: A Comprehensive Guide
Are you struggling with Lewis dot structures? Do practice worksheets leave you feeling more confused than enlightened? Fear not! This comprehensive guide will not only provide answers to common Lewis dot structure practice worksheet questions but also delve deep into the concepts, offering clear explanations and valuable tips to master this crucial chemistry skill. We'll cover everything from basic structures to more complex molecules, ensuring you gain a solid understanding and confidence in tackling any Lewis dot structure problem.
Understanding Lewis Dot Structures: The Fundamentals
Before we jump into the answers, let's solidify our understanding of the fundamental principles behind Lewis dot structures. These structures, also known as electron dot diagrams, visually represent the valence electrons of atoms and how they bond to form molecules. Valence electrons are the electrons in the outermost shell of an atom, and they are the key players in chemical bonding.
Key Concepts:
-
Valence Electrons: The number of valence electrons determines an atom's bonding capacity. You can easily determine the number of valence electrons by looking at the group number of the element on the periodic table (for main group elements). For example, elements in Group 1 have one valence electron, Group 2 elements have two, and so on.
-
Octet Rule: This rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight valence electrons (like a noble gas). There are exceptions, especially for elements in periods beyond the second.
-
Lone Pairs and Bonding Pairs: Valence electrons are represented as dots around the atom's symbol. A lone pair is a pair of electrons not involved in bonding, while a bonding pair (or shared pair) is a pair of electrons shared between two atoms to form a covalent bond.
-
Formal Charge: This concept helps determine the most likely Lewis structure for a molecule, particularly those with multiple possible arrangements. It represents the difference between the number of valence electrons an atom has in its neutral state and the number of electrons assigned to it in the Lewis structure. A structure with the lowest formal charges on each atom is generally preferred.
Tackling Common Lewis Dot Structure Worksheet Questions
Now, let's delve into some common types of problems found in Lewis dot structure practice worksheets, providing step-by-step solutions and explanations.
Example 1: Simple Molecules – Drawing the Lewis Structure of Water (H₂O)
-
Count Valence Electrons: Hydrogen (H) has 1 valence electron, and Oxygen (O) has 6. Total: 1(2) + 6 = 8 valence electrons.
-
Central Atom: Oxygen is the least electronegative atom and becomes the central atom.
-
Connect Atoms: Connect the hydrogen atoms to the oxygen atom with single bonds (each bond uses 2 electrons).
-
Distribute Remaining Electrons: Place the remaining 4 electrons as lone pairs around the oxygen atom.
Result: The Lewis structure of water shows oxygen with two lone pairs and two single bonds to hydrogen atoms.
Example 2: Molecules with Multiple Bonds – Drawing the Lewis Structure of Carbon Dioxide (CO₂)
-
Count Valence Electrons: Carbon (C) has 4 valence electrons, and Oxygen (O) has 6 each. Total: 4 + 6(2) = 16 valence electrons.
-
Central Atom: Carbon is the central atom.
-
Connect Atoms: Connect the oxygen atoms to the carbon atom with single bonds (using 4 electrons).
-
Distribute Remaining Electrons: Place the remaining 12 electrons around the oxygen atoms to satisfy the octet rule. However, this leaves carbon with only four electrons. To satisfy the octet rule for carbon, convert two lone pairs from the oxygen atoms into double bonds with carbon.
Result: The Lewis structure of carbon dioxide shows carbon double-bonded to each oxygen atom.
Example 3: Molecules with Resonance – Drawing the Lewis Structure of Ozone (O₃)
Ozone presents a challenge because multiple valid Lewis structures can be drawn. This phenomenon is known as resonance.
-
Count Valence Electrons: Each oxygen atom has 6 valence electrons. Total: 6(3) = 18 valence electrons.
-
Connect Atoms: Arrange the oxygen atoms linearly, with one in the center. Connect them with single bonds (using 4 electrons).
-
Distribute Remaining Electrons: Distribute the remaining 14 electrons to satisfy the octet rule. However, you'll quickly realize that you can achieve this in multiple ways, creating several possible structures with a double bond and a single bond alternating between the oxygen atoms.
Result: Ozone has resonance structures, implying that the actual structure is a hybrid of these structures. The double bond is delocalized across the molecule.
Example 4: Polyatomic Ions – Drawing the Lewis Structure of Nitrate Ion (NO₃⁻)
Polyatomic ions require an extra step to account for the charge.
-
Count Valence Electrons: Nitrogen (N) has 5, Oxygen (O) has 6 each, and there's an extra electron due to the -1 charge. Total: 5 + 6(3) + 1 = 24 valence electrons.
-
Central Atom: Nitrogen is the central atom.
-
Connect Atoms: Connect the oxygen atoms to the nitrogen atom with single bonds (using 6 electrons).
-
Distribute Remaining Electrons: Distribute the remaining 18 electrons (24 - 6 = 18) to satisfy the octet rule. Similar to ozone, resonance structures exist to achieve the best representation of electron distribution.
Result: The nitrate ion has resonance structures, showing the delocalization of electrons across the molecule.
Example 5: Exceptions to the Octet Rule – Drawing the Lewis Structure of Boron Trifluoride (BF₃)
Boron is a notable exception to the octet rule.
-
Count Valence Electrons: Boron (B) has 3, and Fluorine (F) has 7 each. Total: 3 + 7(3) = 24 valence electrons.
-
Central Atom: Boron is the central atom.
-
Connect Atoms: Connect each fluorine atom to boron with a single bond (using 6 electrons).
-
Distribute Remaining Electrons: Distribute the remaining 18 electrons (24 - 6 = 18) to the fluorine atoms, which achieve octets. Boron, however, only has six valence electrons.
Result: Boron trifluoride is an exception to the octet rule; boron has only six valence electrons.
Advanced Tips and Tricks for Mastering Lewis Dot Structures
-
Practice Regularly: The key to mastering Lewis dot structures is consistent practice. The more problems you work through, the better you'll become at recognizing patterns and applying the rules efficiently.
-
Use Online Resources: Numerous online resources, including interactive simulations and tutorials, can help reinforce your understanding.
-
Check Your Work: Always double-check your work by verifying that you've accounted for all the valence electrons and that each atom (except for exceptions) satisfies the octet rule. Calculate formal charges to ensure the most stable structure is represented.
-
Don't Be Afraid to Make Mistakes: Mistakes are a valuable part of the learning process. If you make a mistake, analyze where you went wrong, and try again.
Beyond the Worksheet: Applying Lewis Dot Structures
Understanding Lewis dot structures is crucial for grasping many key chemistry concepts. They are fundamental to understanding:
-
Molecular Geometry: Lewis structures provide a foundation for predicting the three-dimensional arrangement of atoms in a molecule using concepts like VSEPR theory (Valence Shell Electron Pair Repulsion).
-
Bonding Theories: They help to visualize the different types of chemical bonds: covalent, ionic, and coordinate covalent bonds.
-
Polarity of Molecules: By examining the distribution of electrons, you can determine whether a molecule is polar or nonpolar.
-
Predicting Reactions: Lewis structures can help predict the reactivity of molecules based on their electron configuration.
This comprehensive guide provides a robust foundation for understanding and mastering Lewis dot structures. Remember that consistent practice and a thorough understanding of the underlying concepts are key to success. By diligently working through practice problems and actively engaging with the material, you'll confidently navigate the world of Lewis dot structures and their applications in chemistry.
Latest Posts
Latest Posts
-
Rusting Iron Chemical Or Physical Change
Mar 31, 2025
-
Is A Change In Color A Chemical Change
Mar 31, 2025
-
The Atomic Mass Is Equal To The Number Of
Mar 31, 2025
-
What Is The Mode Of Nutrition For Fungi
Mar 31, 2025
-
What Happens To An Animal Cell In A Isotonic Solution
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Lewis Dot Structure Practice Worksheet Answers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
