Liquid Hydrogen Peroxide Decomposes To Form Water And Oxygen Gas
Muz Play
Mar 15, 2025 · 6 min read
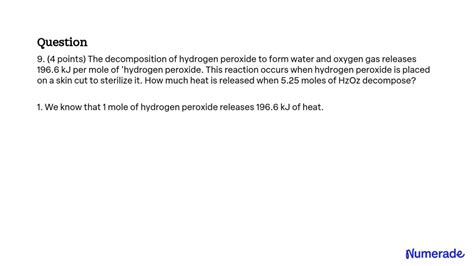
Table of Contents
Liquid Hydrogen Peroxide: Decomposition into Water and Oxygen Gas
Hydrogen peroxide (H₂O₂) is a chemical compound with a multitude of applications, from bleaching agents to rocket propellant. While its versatility is undeniable, understanding its properties, particularly its decomposition, is crucial for safe and effective utilization. This article delves deep into the decomposition of liquid hydrogen peroxide, exploring the reaction mechanism, influencing factors, and practical implications.
Understanding Hydrogen Peroxide's Chemical Structure and Properties
Before examining the decomposition, it's vital to understand the inherent nature of hydrogen peroxide itself. It's a slightly viscous liquid at room temperature, appearing colorless and possessing a characteristic pungent odor. Its molecular structure features two oxygen atoms bonded together, each also bonded to a hydrogen atom. This structure, while seemingly simple, grants it potent oxidizing properties. The oxygen-oxygen bond is relatively weak, making it susceptible to decomposition. This inherent instability is at the heart of its reactivity and the subject of this detailed exploration.
The Instability of the O-O Bond: A Key Factor in Decomposition
The relatively weak oxygen-oxygen bond is the driving force behind hydrogen peroxide's decomposition. This bond is readily broken, leading to the formation of water and oxygen gas. The instability is further enhanced by several factors which will be discussed in detail below. This inherent instability is why hydrogen peroxide is usually stored in dark, cool places to minimize decomposition.
The Decomposition Reaction: A Detailed Look
The decomposition of liquid hydrogen peroxide into water and oxygen gas is an exothermic reaction, meaning it releases heat. The chemical equation representing this process is straightforward:
2H₂O₂ → 2H₂O + O₂
This equation shows that two molecules of hydrogen peroxide break down to form two molecules of water and one molecule of oxygen gas. The released oxygen gas is responsible for many of the applications of hydrogen peroxide, from its use as a disinfectant to its role as an oxidizer in rocket fuel.
The Mechanism of Decomposition: A Step-by-Step Analysis
While the overall reaction is simple, the decomposition mechanism is more complex. It often involves a series of steps, often catalyzed by various substances. One common mechanism involves the formation of free radicals:
-
Initiation: The decomposition can be initiated by various factors, including heat, light, or the presence of catalysts (discussed later). This step often involves the homolytic cleavage of the O-O bond, creating two hydroxyl radicals (•OH).
-
Propagation: The hydroxyl radicals then react with more hydrogen peroxide molecules, generating more hydroxyl radicals and water molecules in a chain reaction:
- •OH + H₂O₂ → H₂O + •OOH
- •OOH + H₂O₂ → H₂O + O₂ + •OH
-
Termination: The chain reaction eventually terminates when two radicals combine, for instance:
- •OH + •OH → H₂O₂
- •OH + •OOH → H₂O + O₂
This chain reaction nature explains why the decomposition can be quite rapid once initiated. The speed of the reaction is highly dependent on the concentration of radicals and the presence of catalysts.
Factors Influencing the Decomposition Rate
Several factors significantly impact the rate at which hydrogen peroxide decomposes:
1. Temperature: The Heat Factor
Higher temperatures accelerate the decomposition rate. The increased kinetic energy of the molecules at higher temperatures leads to more frequent and energetic collisions, increasing the probability of bond breakage and the initiation of the chain reaction. This is why hydrogen peroxide is typically stored at low temperatures.
2. Catalysts: Speeding Up the Process
Certain substances, known as catalysts, dramatically increase the decomposition rate without being consumed themselves. These catalysts often work by lowering the activation energy required for the reaction to occur. Common catalysts include:
- Transition metal ions: Ions of metals such as manganese, iron, copper, and silver are particularly effective catalysts. They often participate in redox reactions within the decomposition mechanism.
- Enzymes: Certain enzymes, particularly catalases, found in living organisms, are highly efficient catalysts for the decomposition of hydrogen peroxide. Catalases play a crucial role in protecting cells from the damaging effects of hydrogen peroxide.
- Surfaces: The surface area of the container holding hydrogen peroxide can also influence the decomposition rate. Rough surfaces can provide sites for the reaction to occur more readily.
3. Light: A Photochemical Trigger
Exposure to light, particularly ultraviolet (UV) light, can also initiate and accelerate the decomposition of hydrogen peroxide. UV light provides the energy needed to break the O-O bond, initiating the free radical chain reaction. This is why hydrogen peroxide is often stored in dark or amber-colored bottles.
4. Concentration: The Role of Purity
The concentration of hydrogen peroxide itself also influences the decomposition rate. Higher concentrations generally decompose faster due to the increased probability of collisions between hydrogen peroxide molecules.
5. Impurities: Unwanted Accelerants
The presence of impurities in hydrogen peroxide can also accelerate its decomposition. These impurities might act as catalysts or contribute to the generation of free radicals. Therefore, high-purity hydrogen peroxide is essential for stability and long shelf life.
Practical Implications and Applications of Hydrogen Peroxide Decomposition
The decomposition of hydrogen peroxide has numerous practical applications and implications:
1. Disinfectant and Antiseptic Properties
The release of oxygen during decomposition contributes to hydrogen peroxide's disinfecting and antiseptic properties. The nascent oxygen is highly reactive and can effectively kill bacteria, viruses, and fungi.
2. Rocket Propulsion: A Powerful Oxidizer
The decomposition of hydrogen peroxide releases a large amount of energy, making it a suitable propellant for rockets. Concentrated hydrogen peroxide, often catalyzed, can be used to generate hot steam and oxygen, providing thrust.
3. Bleaching Agent: Oxidation Power
The oxidizing power of hydrogen peroxide, a consequence of its decomposition and the release of oxygen, makes it a widely used bleaching agent in various industries, including textiles and paper production.
4. Chemical Synthesis: A Versatile Reactant
Hydrogen peroxide's decomposition can be harnessed in chemical synthesis to generate reactive oxygen species, useful in various chemical reactions.
5. Waste Treatment: Environmental Applications
Hydrogen peroxide decomposition can be utilized in environmental remediation. Its oxidizing capabilities can break down pollutants and contaminants, contributing to waste water treatment.
Safety Precautions: Handling Hydrogen Peroxide
Due to its reactive nature and potential for decomposition, handling hydrogen peroxide requires caution:
- Storage: Store hydrogen peroxide in cool, dark places, away from direct sunlight and heat sources.
- Containers: Use appropriate containers made of materials resistant to corrosion.
- Contact: Avoid direct contact with skin and eyes; use appropriate personal protective equipment (PPE).
- Mixing: Never mix hydrogen peroxide with other chemicals without proper knowledge and safety precautions, as this may result in violent reactions.
Conclusion: A Powerful yet Delicate Compound
The decomposition of liquid hydrogen peroxide into water and oxygen gas is a fundamental chemical process with far-reaching implications. Understanding the reaction mechanism, influencing factors, and safety precautions is crucial for its safe and effective use in diverse applications. From its role as a disinfectant to its use in rocket propulsion, the controlled decomposition of hydrogen peroxide continues to be an important aspect of chemistry and its various fields. The inherent instability of this compound, while requiring careful handling, is also the source of its remarkable versatility and effectiveness in a wide range of applications. Further research continues to explore the intricacies of this decomposition process and find new ways to harness its power while mitigating potential risks.
Latest Posts
Latest Posts
-
Fourier Transform Of A Differential Equation
Mar 15, 2025
-
Which One Neutral Charge Proton Or Neutron
Mar 15, 2025
-
A Solids Volume And Shape Is Defintie
Mar 15, 2025
-
How Many Protons Does Iodine Have
Mar 15, 2025
-
If The Finches On The Galapagos Islands
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Liquid Hydrogen Peroxide Decomposes To Form Water And Oxygen Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
