List Of Elements With Protons Neutrons And Electrons
Muz Play
Mar 22, 2025 · 7 min read
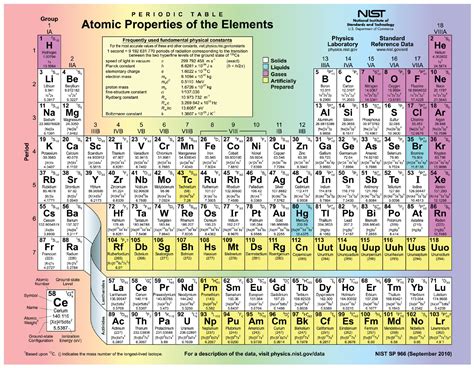
Table of Contents
A Comprehensive Guide to Elements, Protons, Neutrons, and Electrons
Understanding the fundamental building blocks of matter is crucial to grasping the complexities of chemistry and physics. This article delves deep into the composition of elements, focusing on the interplay of protons, neutrons, and electrons. We'll explore how these subatomic particles define an element's properties and how to determine their numbers for any given element.
Understanding Subatomic Particles
Before diving into specific elements, let's establish a firm understanding of the three key subatomic particles:
Protons:
- Charge: +1 (positive)
- Mass: Approximately 1 atomic mass unit (amu)
- Location: Nucleus
- Defining Characteristic: The number of protons in an atom's nucleus defines its atomic number and thus, its identity as a specific element. All atoms of a given element have the same number of protons. For example, all hydrogen atoms have one proton, all helium atoms have two, and so on.
Neutrons:
- Charge: 0 (neutral)
- Mass: Approximately 1 atomic mass unit (amu)
- Location: Nucleus
- Isotopes and Mass Number: The number of neutrons in an atom can vary, even within the same element. Atoms of the same element with different numbers of neutrons are called isotopes. The mass number of an atom is the sum of its protons and neutrons.
Electrons:
- Charge: -1 (negative)
- Mass: Approximately 1/1836 amu (negligible compared to protons and neutrons)
- Location: Electron cloud surrounding the nucleus
- Chemical Behavior: Electrons determine an atom's chemical behavior. The arrangement of electrons in energy levels (shells) dictates how an atom will interact with other atoms to form chemical bonds. The outermost electrons, known as valence electrons, are particularly important in this regard.
Determining the Number of Protons, Neutrons, and Electrons
For a neutral atom (an atom with no overall charge), the number of protons equals the number of electrons. However, the number of neutrons can vary.
Here's how to determine the number of each subatomic particle:
-
Atomic Number (Z): This is the number of protons in the nucleus of an atom. It's unique to each element and is usually found on the periodic table.
-
Mass Number (A): This is the total number of protons and neutrons in the nucleus. It's often written as a superscript to the left of the element symbol (e.g., ¹²C, where 12 is the mass number).
-
Calculating Neutrons: To find the number of neutrons, subtract the atomic number (number of protons) from the mass number: Number of neutrons = Mass number (A) - Atomic number (Z)
-
Electrons in a Neutral Atom: In a neutral atom, the number of electrons equals the number of protons (atomic number).
Example: Carbon-12 (¹²C)
- Atomic Number (Z): 6 (found on the periodic table)
- Mass Number (A): 12 (given)
- Number of Protons: 6
- Number of Neutrons: 12 - 6 = 6
- Number of Electrons: 6 (since it's a neutral atom)
A List of Elements with Protons, Neutrons, and Electrons (Selected Examples)
It's impossible to list all elements and their isotopes here, as there are over 100 elements and countless isotopes. However, we can examine several common elements to illustrate the concepts:
| Element | Symbol | Atomic Number (Z) | Mass Number (A) (most common isotope) | Protons | Neutrons | Electrons (neutral atom) |
|---|---|---|---|---|---|---|
| Hydrogen | H | 1 | 1 | 1 | 0 | 1 |
| Helium | He | 2 | 4 | 2 | 2 | 2 |
| Lithium | Li | 3 | 7 | 3 | 4 | 3 |
| Beryllium | Be | 4 | 9 | 4 | 5 | 4 |
| Boron | B | 5 | 11 | 5 | 6 | 5 |
| Carbon | C | 6 | 12 | 6 | 6 | 6 |
| Nitrogen | N | 7 | 14 | 7 | 7 | 7 |
| Oxygen | O | 8 | 16 | 8 | 8 | 8 |
| Fluorine | F | 9 | 19 | 9 | 10 | 9 |
| Neon | Ne | 10 | 20 | 10 | 10 | 10 |
| Sodium | Na | 11 | 23 | 11 | 12 | 11 |
| Magnesium | Mg | 12 | 24 | 12 | 12 | 12 |
| Aluminum | Al | 13 | 27 | 13 | 14 | 13 |
| Silicon | Si | 14 | 28 | 14 | 14 | 14 |
| Phosphorus | P | 15 | 31 | 15 | 16 | 15 |
| Sulfur | S | 16 | 32 | 16 | 16 | 16 |
| Chlorine | Cl | 17 | 35 | 17 | 18 | 17 |
| Argon | Ar | 18 | 40 | 18 | 22 | 18 |
| Potassium | K | 19 | 39 | 19 | 20 | 19 |
| Calcium | Ca | 20 | 40 | 20 | 20 | 20 |
| Iron | Fe | 26 | 56 | 26 | 30 | 26 |
| Copper | Cu | 29 | 63 | 29 | 34 | 29 |
| Gold | Au | 79 | 197 | 79 | 118 | 79 |
| Lead | Pb | 82 | 207 | 82 | 125 | 82 |
| Uranium | U | 92 | 238 | 92 | 146 | 92 |
Important Note: The mass number shown above represents the most abundant isotope for each element. Many elements have multiple isotopes, each with a different number of neutrons.
Isotopes: Variations on a Theme
As mentioned earlier, isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. This means they have the same atomic number but different mass numbers. Some isotopes are stable, while others are radioactive, meaning they undergo radioactive decay, emitting particles and energy.
Examples of Isotopes:
- Carbon: Carbon-12 (¹²C), Carbon-13 (¹³C), Carbon-14 (¹⁴C). ¹⁴C is a radioactive isotope used in carbon dating.
- Hydrogen: Protium (¹H), Deuterium (²H), Tritium (³H). Deuterium and tritium are isotopes of hydrogen with one and two neutrons, respectively. Tritium is radioactive.
Ions: Charged Atoms
Atoms can gain or lose electrons to become ions. Ions are atoms with a net electrical charge.
- Cations: Positively charged ions formed when an atom loses electrons.
- Anions: Negatively charged ions formed when an atom gains electrons.
The number of protons remains the same in an ion, but the number of electrons changes, resulting in a net positive or negative charge. For example, a sodium ion (Na⁺) has 11 protons and 10 electrons, giving it a +1 charge.
The Periodic Table and Elemental Properties
The periodic table is a powerful tool for organizing elements based on their atomic number and recurring chemical properties. The arrangement of elements reflects the electronic structure of their atoms and helps predict their reactivity and other characteristics.
By understanding the relationship between the number of protons, neutrons, and electrons, we can better interpret the periodic table and predict how different elements will interact with each other.
Conclusion
The composition of atoms in terms of protons, neutrons, and electrons forms the fundamental basis of chemistry. By understanding the atomic number, mass number, and the behavior of isotopes and ions, we can gain a deeper appreciation of the properties and interactions of matter. This knowledge is crucial for advancements in various fields, including medicine, materials science, and energy production. Further exploration of quantum mechanics provides even deeper insight into the complexities of atomic structure. This article offers a foundational understanding, serving as a springboard for more advanced studies in atomic and nuclear physics.
Latest Posts
Latest Posts
-
Determinants Of Price Elasticity Of Supply
Mar 22, 2025
-
What Is Stronger Ionic Or Covalent Bonds
Mar 22, 2025
-
Solve The System Of Linear Equations Algebraically
Mar 22, 2025
-
What Is The Difference Between Actual Yield And Theoretical Yield
Mar 22, 2025
-
Compared With Solid Ionic Compounds Solid Molecular Compounds Generally
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about List Of Elements With Protons Neutrons And Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
