What Is The Difference Between Actual Yield And Theoretical Yield
Muz Play
Mar 22, 2025 · 5 min read
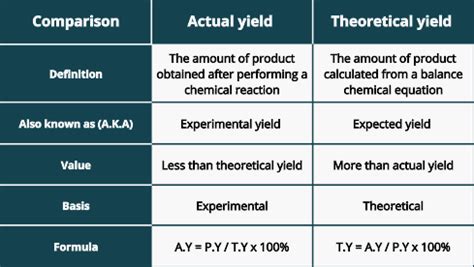
Table of Contents
What's the Difference Between Actual Yield and Theoretical Yield? A Comprehensive Guide
Understanding the difference between actual yield and theoretical yield is crucial in chemistry, particularly in stoichiometry and experimental design. While both terms relate to the quantity of product obtained from a chemical reaction, they represent different aspects of the process, highlighting the gap between idealized calculations and real-world outcomes. This comprehensive guide will delve into the definitions, calculations, factors influencing the discrepancy, and the significance of understanding this difference in various applications.
Understanding Theoretical Yield
Theoretical yield represents the maximum possible amount of product that can be formed from a given amount of reactant(s), assuming 100% conversion of the limiting reactant. It's a purely calculated value based on the stoichiometric ratios in the balanced chemical equation. This calculation doesn't account for real-world limitations like incomplete reactions, side reactions, or loss of product during the process.
Calculating Theoretical Yield
Calculating theoretical yield involves these steps:
-
Balance the chemical equation: Ensure you have a correctly balanced equation representing the reaction. This establishes the molar ratios between reactants and products.
-
Identify the limiting reactant: Determine which reactant is present in the smallest stoichiometric amount, limiting the amount of product that can be formed.
-
Calculate moles of limiting reactant: Convert the given mass (or volume) of the limiting reactant into moles using its molar mass.
-
Use stoichiometry to calculate moles of product: Employ the mole ratio from the balanced equation to determine the moles of product formed from the moles of limiting reactant.
-
Convert moles of product to grams: Use the molar mass of the product to convert the moles of product calculated in step 4 into grams, which represents the theoretical yield.
Example: Consider the reaction: 2H₂ + O₂ → 2H₂O
If we start with 2 moles of H₂ and 1 mole of O₂, the limiting reactant is H₂. The mole ratio of H₂ to H₂O is 2:2 (or 1:1). Therefore, 2 moles of H₂ will produce 2 moles of H₂O. If the molar mass of H₂O is 18 g/mol, the theoretical yield of H₂O is 2 moles * 18 g/mol = 36 g.
Understanding Actual Yield
Actual yield, on the other hand, is the actual amount of product obtained from a chemical reaction in a laboratory setting. This is a measured value, obtained experimentally after the reaction is complete and the product is isolated and purified. It's always less than or equal to the theoretical yield.
Measuring Actual Yield
Measuring actual yield involves carefully isolating and purifying the product. This typically involves techniques like filtration, extraction, recrystallization, and drying. The purified product is then weighed to determine its mass. The accuracy of the actual yield depends significantly on the precision and efficiency of these separation and purification techniques.
The Difference: Why Actual Yield is Often Less Than Theoretical Yield
The difference between actual and theoretical yield arises from various factors that inevitably impact real-world reactions:
-
Incomplete reactions: Not all reactant molecules may react to form the desired product. Some may remain unreacted, leading to a lower yield.
-
Side reactions: Unwanted reactions may occur simultaneously, consuming some reactants and producing byproducts, reducing the amount of desired product formed.
-
Equilibria: Many reactions are reversible, reaching a state of equilibrium where both reactants and products are present. The actual yield is limited by the equilibrium position.
-
Loss of product during isolation and purification: Product may be lost during the separation and purification steps. Some product may stick to glassware, be lost in filtrates, or decompose during purification.
-
Impurities in reactants: The presence of impurities in reactants can interfere with the reaction and reduce the yield.
-
Experimental errors: Human error in measuring reactants, controlling reaction conditions, or performing separation techniques can lead to lower yields.
Calculating Percent Yield
The relationship between actual and theoretical yield is often expressed as the percent yield:
Percent Yield = (Actual Yield / Theoretical Yield) x 100%
Percent yield provides a quantitative measure of the reaction's efficiency. A high percent yield (close to 100%) indicates a highly efficient reaction, while a low percent yield suggests significant losses or inefficiencies in the process.
Significance of Understanding Actual vs. Theoretical Yield
Understanding the difference between actual and theoretical yield is essential in numerous contexts:
-
Industrial Processes: In industrial chemistry, maximizing yield is critical for economic viability. Understanding factors that reduce yield allows for process optimization and cost reduction.
-
Research and Development: In research laboratories, yield is an important indicator of reaction success and efficiency. Low yields might necessitate modifying reaction conditions, exploring alternative synthetic routes, or improving purification techniques.
-
Quality Control: In pharmaceutical and other industries, consistent and high yields are essential for quality control and ensuring product purity.
-
Environmental Impact: High yields minimize waste generation, contributing to more environmentally friendly chemical processes.
Advanced Considerations and Applications
The concept extends beyond simple stoichiometric calculations. In more complex reactions, factors like reaction kinetics, thermodynamics, and catalyst efficiency play significant roles in determining the actual yield.
Kinetics: The rate at which a reaction proceeds influences the extent of completion. Slower reactions may not reach completion within a reasonable timeframe, resulting in a lower actual yield.
Thermodynamics: The spontaneity and equilibrium constant of a reaction determine the theoretical yield at equilibrium. Reactions with unfavorable thermodynamics may exhibit low yields, regardless of reaction time.
Catalyst Effects: Catalysts accelerate reaction rates, potentially improving the yield by facilitating the formation of the desired product.
Conclusion: Bridging the Gap Between Theory and Practice
The difference between actual and theoretical yield underscores the gap between idealized chemical calculations and the realities of performing reactions in the laboratory or industrial settings. While theoretical yield provides a valuable benchmark, actual yield reflects the practical efficiency of the process. Understanding the factors that contribute to this discrepancy is crucial for optimizing reaction conditions, improving experimental techniques, and maximizing product yield in diverse applications across science and industry. By carefully considering the factors discussed above and meticulously implementing experimental protocols, chemists can strive to bridge the gap between theory and practice and achieve higher yields in their endeavors.
Latest Posts
Latest Posts
-
Is Carbonate Ion A Strong Base
Mar 23, 2025
-
What Is A Family Of Elements
Mar 23, 2025
-
Unidades De Medida En Estados Unidos
Mar 23, 2025
-
Which Is The Electron Configuration For Lithium
Mar 23, 2025
-
Integration By Parts Examples With Solutions
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Actual Yield And Theoretical Yield . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
