Lithium Aluminum Hydride Reduction Of Ester
Muz Play
Mar 23, 2025 · 6 min read
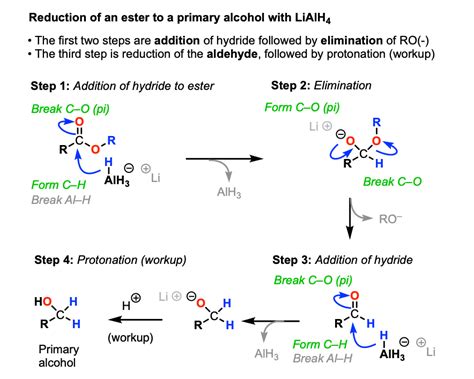
Table of Contents
Lithium Aluminum Hydride Reduction of Esters: A Comprehensive Guide
The reduction of esters using lithium aluminum hydride (LiAlH₄) is a fundamental reaction in organic chemistry, offering a powerful and versatile method for synthesizing primary alcohols. This process, characterized by its strong reducing capabilities, efficiently cleaves the ester's carbonyl group, converting it into a hydroxyl group while simultaneously reducing the alkyl portion to an alkane. This detailed guide will explore the mechanism, applications, advantages, limitations, and safety precautions associated with this vital transformation.
Understanding the Reaction Mechanism
The reduction of esters by LiAlH₄ proceeds through a series of nucleophilic attacks and hydride transfers. The reaction is typically carried out in anhydrous ethereal solvents like diethyl ether or tetrahydrofuran (THF), which are crucial for solvating the LiAlH₄ and facilitating the reaction. The mechanism can be broken down into several key steps:
Step 1: Nucleophilic Attack
The hydride ion (H⁻), a potent nucleophile, from LiAlH₄ attacks the electrophilic carbonyl carbon of the ester. This attack results in the formation of a tetrahedral intermediate. The negatively charged oxygen atom in this intermediate is stabilized by complexation with the aluminum atom.
Step 2: Elimination of Alkoxy Group
The alkoxy group (RO⁻) leaves as an alkoxide ion. This step is facilitated by the electron-withdrawing effect of the carbonyl group, which weakens the carbon-oxygen bond. The resulting intermediate is an aldehyde.
Step 3: Second Hydride Attack and Formation of Alkoxide
The newly formed aldehyde is highly reactive and immediately undergoes a second nucleophilic attack by another hydride ion from LiAlH₄. This attack results in the formation of an alkoxide intermediate.
Step 4: Acidic Workup
The reaction is completed by an acidic workup. This involves the careful addition of a dilute acid, such as dilute sulfuric acid or hydrochloric acid. This step protonates the alkoxide ion, converting it into the corresponding primary alcohol. The aluminum salts formed during the reaction are removed via filtration or extraction.
Factors Affecting the Reaction
Several factors influence the efficiency and outcome of the LiAlH₄ reduction of esters. These include:
Solvent Selection:
The choice of solvent plays a critical role. Anhydrous conditions are absolutely essential as even traces of water can react violently with LiAlH₄, leading to the evolution of hydrogen gas and potentially dangerous explosions. Diethyl ether and THF are commonly used due to their ability to dissolve both the ester and LiAlH₄.
Temperature Control:
The reaction is generally exothermic. Careful temperature control is necessary to prevent uncontrolled reactions and side products. The reaction is typically carried out at low to moderate temperatures, often below 0°C initially, and then allowed to gradually warm to room temperature.
Stoichiometry:
The stoichiometry of the reaction is crucial. A minimum of four equivalents of LiAlH₄ are required for the complete reduction of one equivalent of ester. Excess LiAlH₄ can be used to ensure complete conversion, but it needs to be carefully quenched during workup.
Steric Hindrance:
Sterically hindered esters may react more slowly than unhindered esters. The bulky groups surrounding the carbonyl group can hinder the nucleophilic attack by the hydride ion, leading to slower reaction rates and potentially lower yields.
Applications of LiAlH₄ Reduction of Esters
The LiAlH₄ reduction of esters has widespread applications in organic synthesis, serving as a critical tool in the preparation of various molecules. Some key applications include:
Synthesis of Primary Alcohols:
This is the primary application of the reaction, providing a direct and efficient route to primary alcohols from various esters. This is invaluable in the synthesis of complex molecules containing alcohol functional groups.
Preparation of Pharmaceuticals:
Many pharmaceuticals contain alcohol functional groups, and LiAlH₄ reduction is frequently used in their synthesis. The precise control and high yields achievable make it a valuable tool in pharmaceutical development.
Synthesis of Natural Products:
The reduction of esters is often a crucial step in the total synthesis of complex natural products containing alcohol moieties. The ability of LiAlH₄ to selectively reduce esters in the presence of other functional groups makes it particularly useful in these syntheses.
Advantages of Using LiAlH₄
Compared to other reducing agents, LiAlH₄ offers several advantages:
- High Reactivity: LiAlH₄ is a powerful reducing agent capable of reducing a wide range of esters efficiently.
- Versatility: It can be used with a variety of esters, regardless of the size or structure of the alkyl or alkoxy groups.
- High Yields: Under optimal conditions, LiAlH₄ reductions typically result in high yields of the desired primary alcohol.
- Selectivity: While it effectively reduces esters, it can be selective in the presence of other functional groups under appropriate conditions.
Limitations of Using LiAlH₄
Despite its advantages, LiAlH₄ has certain limitations:
- Sensitivity to Water and Air: LiAlH₄ is extremely sensitive to moisture and air, requiring anhydrous conditions for optimal results. Contact with water or air can lead to violent reactions.
- Harsh Reaction Conditions: The reaction conditions can be relatively harsh, requiring low temperatures and careful handling of reagents.
- Toxicity: LiAlH₄ is toxic and requires careful handling and disposal. Appropriate safety precautions are essential.
- Difficult Workup: The workup process can be somewhat complex, often requiring careful quenching with acid and subsequent extraction and purification.
Safety Precautions
Working with LiAlH₄ demands rigorous adherence to safety protocols due to its reactivity and toxicity. Key safety precautions include:
- Anhydrous Conditions: All glassware and reagents must be meticulously dried to remove any traces of water.
- Inert Atmosphere: The reaction should be carried out under an inert atmosphere, such as nitrogen or argon, to prevent exposure to air and moisture.
- Protective Equipment: Appropriate personal protective equipment (PPE), including gloves, goggles, and a lab coat, must be worn at all times.
- Careful Addition: LiAlH₄ should be added slowly and cautiously to the reaction mixture to control the exothermic nature of the reaction.
- Careful Quenching: The reaction must be carefully quenched with dilute acid, often with an ice bath, to control the evolution of hydrogen gas.
- Proper Disposal: All waste materials should be disposed of according to proper safety guidelines and regulations.
Alternative Reducing Agents
While LiAlH₄ is a powerful reducing agent, other reagents can also reduce esters, each with its own set of advantages and disadvantages:
- Diborane (B₂H₆): Diborane is a milder reducing agent than LiAlH₄ and may be preferred for more sensitive substrates.
- Sodium Borohydride (NaBH₄): Sodium borohydride is a much milder reducing agent than LiAlH₄ and generally will not reduce esters. It's often used for reducing aldehydes and ketones.
- Other Metal Hydrides: Various other metal hydrides, such as sodium bis(2-methoxyethoxy)aluminum hydride (Red-Al), offer alternative reducing capabilities.
Conclusion
The lithium aluminum hydride reduction of esters is a cornerstone reaction in organic chemistry, offering a versatile and powerful method for synthesizing primary alcohols. Understanding the mechanism, factors influencing the reaction, safety precautions, and potential alternatives allows for the efficient and safe execution of this crucial transformation. Remember, always prioritize safety when working with LiAlH₄ and adhere strictly to established laboratory procedures. The careful planning and execution of this reaction are crucial for obtaining high yields of the desired product. This detailed overview should equip you with the necessary knowledge to confidently approach and execute this essential organic chemistry reaction.
Latest Posts
Latest Posts
-
An Organism That Obtains Its Energy From Sunlight
Mar 24, 2025
-
How Does A Tendon Sheath Differ From A Bursa
Mar 24, 2025
-
Is Vegetable Oil A Pure Substance Or A Mixture
Mar 24, 2025
-
Is Tap Water Homogeneous Or Heterogeneous
Mar 24, 2025
-
What Is The Electron Configuration For Boron
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Lithium Aluminum Hydride Reduction Of Ester . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
