What Is The Electron Configuration For Boron
Muz Play
Mar 24, 2025 · 5 min read
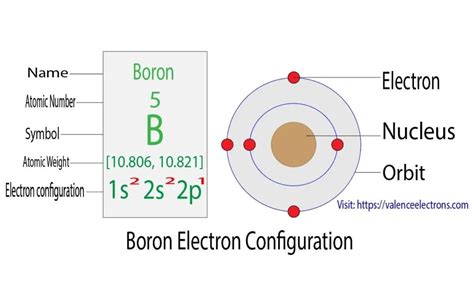
Table of Contents
What is the Electron Configuration for Boron? A Deep Dive into Atomic Structure
Boron, a metalloid element crucial in various applications, holds a fascinating position in the periodic table. Understanding its electron configuration is key to comprehending its chemical properties and behavior. This comprehensive guide delves deep into the electron configuration of boron, exploring its implications and relating it to broader concepts in atomic structure and chemical bonding.
Understanding Electron Configuration
Before we delve into boron specifically, let's establish a foundational understanding of electron configuration. Electron configuration describes the arrangement of electrons in an atom's electron shells and subshells. This arrangement dictates an atom's chemical behavior and its interactions with other atoms. It follows specific rules governed by quantum mechanics, primarily the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
- Aufbau principle: Electrons fill orbitals starting from the lowest energy level and progressing upwards.
- Hund's rule: Electrons fill orbitals individually before pairing up within the same subshell.
- Pauli exclusion principle: Each orbital can hold a maximum of two electrons, each with opposite spins.
These principles guide us in predicting the electron configuration of any element. The notation used typically represents the principal energy level (n), the subshells (s, p, d, f), and the number of electrons in each subshell. For instance, 1s² indicates two electrons in the 1s subshell.
Determining Boron's Electron Configuration
Boron (B) has an atomic number of 5, meaning it possesses 5 protons and, in its neutral state, 5 electrons. Using the principles outlined above, we can systematically determine its electron configuration.
The lowest energy level (n=1) has only one subshell, the s subshell, which can hold a maximum of two electrons. Therefore, the first two electrons of boron fill the 1s orbital.
The next energy level (n=2) contains two subshells: the s subshell and the p subshell. The 2s subshell, like the 1s, can hold two electrons. These two electrons fill the 2s orbital.
This leaves one electron remaining. This electron will occupy the 2p subshell. The 2p subshell has three orbitals, each capable of holding two electrons, but according to Hund's rule, this single electron occupies one of the 2p orbitals individually.
Therefore, the complete electron configuration of boron is 1s²2s²2p¹.
Visualizing Boron's Electron Configuration
Imagine the atom's nucleus at the center. Around the nucleus, we have energy levels (shells). The first shell (n=1) contains the 1s subshell, a spherical orbital holding two electrons. The second shell (n=2) contains the 2s subshell (also spherical) and the 2p subshell, consisting of three dumbbell-shaped orbitals oriented along different axes (px, py, pz).
In boron's case, the 1s and 2s subshells are completely filled, each with two electrons. The 2p subshell has only one electron, residing in one of its three orbitals. This lone electron in the 2p orbital significantly influences boron's chemical reactivity.
Boron's Chemical Properties and Electron Configuration
Boron's electron configuration directly impacts its chemical properties. The presence of three valence electrons (the electrons in the outermost shell) makes boron relatively reactive. It readily forms covalent bonds by sharing these valence electrons with other atoms to achieve a more stable electron configuration, often resembling that of a noble gas.
Boron's tendency to form covalent bonds is evident in its numerous compounds. It does not readily form ionic bonds due to its relatively high ionization energy—the energy required to remove an electron. This high ionization energy makes it difficult to completely lose its three valence electrons to form a +3 ion.
Examples of Boron's Chemical Behavior:
-
Boron Trifluoride (BF₃): Boron shares its three valence electrons with three fluorine atoms, forming three covalent bonds and achieving a stable octet (eight electrons in the outermost shell) for each fluorine atom. However, boron itself only has six electrons in its valence shell, making it an electron-deficient compound.
-
Borates: Boron readily forms various borate compounds with oxygen. These compounds exhibit complex structures due to boron's ability to form both three-coordinate (trigonal planar) and four-coordinate (tetrahedral) bonds.
-
Boranes: These compounds consist of boron and hydrogen and demonstrate a unique range of structures, often featuring boron atoms with fewer than eight valence electrons. This electron deficiency leads to unusual bonding characteristics.
Electron Configuration and Periodicity
Boron's position in the periodic table—group 13 (or IIIA) and period 2—is directly related to its electron configuration. Elements within the same group share similar valence electron configurations, leading to similar chemical properties. For instance, aluminum (Al), located directly below boron, also has three valence electrons (3s²3p¹) and exhibits some similar chemical behaviors, though the properties differ due to the higher principal quantum number.
The period number indicates the highest principal quantum number (n) of the electrons in the ground state. Boron's period 2 placement implies that its highest occupied energy level is n=2.
Advanced Concepts and Applications
The understanding of boron's electron configuration extends beyond basic chemical behavior. It plays a significant role in:
-
Spectroscopy: The specific arrangement of electrons determines the energy levels within the atom. This allows us to predict the wavelengths of light absorbed or emitted during electronic transitions, facilitating spectroscopic analysis.
-
Materials Science: Boron's unique electronic structure and chemical properties are exploited in the design of advanced materials, including high-strength lightweight composites, semiconductors, and superhard materials.
-
Nuclear Physics: Boron isotopes, particularly boron-10, are used in neutron detectors and nuclear medicine due to their high neutron absorption cross-sections. This is related to their nuclear structure, which is indirectly influenced by electron configuration.
Conclusion
The seemingly simple electron configuration of boron—1s²2s²2p¹—holds the key to understanding its diverse chemical behavior and its significance in various scientific and technological applications. By appreciating the principles governing electron arrangement and its implications for atomic interactions, we can gain a deeper appreciation for the fascinating world of chemistry and the role of this seemingly simple element. This detailed analysis demonstrates how a fundamental understanding of electron configuration can unlock a wealth of knowledge about the properties and applications of elements across the periodic table. The study of boron’s electron configuration serves as a perfect stepping stone to further explore the complexities of atomic structure and chemical bonding.
Latest Posts
Latest Posts
-
How To Solve A Nonlinear Inequality
Mar 25, 2025
-
Pka Of Amino Acid Side Chains
Mar 25, 2025
-
Two Different Isotopes Of An Element Have Different
Mar 25, 2025
-
6 Signs Of A Chemical Reaction
Mar 25, 2025
-
How To Find Ph With Molarity
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Boron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
