Pka Of Amino Acid Side Chains
Muz Play
Mar 25, 2025 · 7 min read
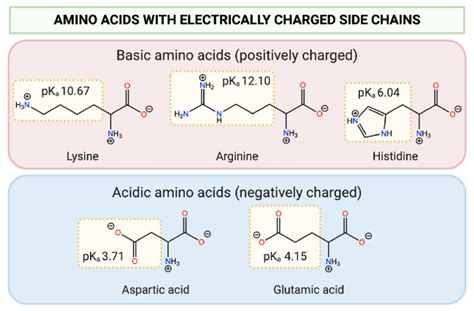
Table of Contents
Understanding the pKa of Amino Acid Side Chains: A Comprehensive Guide
Amino acids, the fundamental building blocks of proteins, possess unique chemical properties largely dictated by their side chains. These side chains, also known as R-groups, exhibit varying degrees of acidity or basicity, quantified by their pKa values. Understanding the pKa of amino acid side chains is crucial for comprehending protein structure, function, and interactions. This comprehensive guide delves into the intricacies of pKa values, their determination, and their significance in biological systems.
What is pKa?
The pKa value represents the acid dissociation constant (Ka) of a weak acid. It's a measure of the tendency of an acid to donate a proton (H+). A lower pKa indicates a stronger acid, meaning it readily releases its proton. Conversely, a higher pKa suggests a weaker acid, less inclined to donate its proton. In the context of amino acids, the pKa refers to the ionization of the side chain's functional group.
pKa = -log₁₀(Ka)
The pKa is not a fixed value; it's influenced by several factors including:
- Temperature: Increased temperature generally lowers pKa.
- Solvent: The polarity and dielectric constant of the solvent significantly impact pKa. Aqueous solutions are the most common context in biological systems.
- Ionic strength: The presence of ions in the solution can alter the pKa.
- Local environment: Within a protein, the microenvironment surrounding the side chain (e.g., proximity to other charged residues, hydrogen bonding) can drastically affect its effective pKa. This deviation from the pKa in solution is often substantial.
pKa Values of Amino Acid Side Chains: A Detailed Look
Amino acids are categorized based on the properties of their side chains: nonpolar, polar uncharged, polar charged (acidic and basic). The pKa values of their side chains significantly influence their behavior within proteins.
Nonpolar Amino Acids
Nonpolar amino acids generally have side chains with pKa values far outside the physiological pH range (approximately pH 7.4). They don't readily ionize under physiological conditions and thus don't significantly contribute to the overall charge of a protein. Examples include:
- Glycine (Gly, G): Lacks a significant side chain, therefore no pKa value associated with the side chain.
- Alanine (Ala, A): Methyl group as a side chain; no ionizable group, hence no relevant pKa.
- Valine (Val, V): Isopropyl group; non-ionizable.
- Leucine (Leu, L): Isobutyl group; non-ionizable.
- Isoleucine (Ile, I): Sec-butyl group; non-ionizable.
- Methionine (Met, M): Thioether group; non-ionizable.
- Proline (Pro, P): Cyclic structure; although it can influence the protein's conformation, its side chain isn't ionizable at physiological pH.
- Phenylalanine (Phe, F): Benzyl group; non-ionizable.
- Tryptophan (Trp, W): Indole group; non-ionizable.
Polar Uncharged Amino Acids
These amino acids possess polar side chains that can participate in hydrogen bonding but typically don't ionize at physiological pH. Their pKa values, if considered, are usually outside the relevant biological range.
- Serine (Ser, S): Hydroxyl group; weakly acidic, pKa around 13-14.
- Threonine (Thr, T): Hydroxyl group; weakly acidic, pKa around 13-14.
- Cysteine (Cys, C): Thiol group; pKa around 8.3. This is an exception; at physiological pH, it can exist as either a thiol (-SH) or a thiolate (-S-), impacting its reactivity and participation in disulfide bond formation.
- Tyrosine (Tyr, Y): Phenolic hydroxyl group; pKa around 10. Its ionization can play a role in certain enzymatic reactions.
- Asparagine (Asn, N): Amide group; non-ionizable at physiological pH.
- Glutamine (Gln, Q): Amide group; non-ionizable at physiological pH.
Polar Charged Amino Acids (Acidic)
These amino acids possess acidic side chains with carboxyl groups that readily ionize at or near physiological pH.
- Aspartic Acid (Asp, D): Carboxyl group; pKa around 3.9. At physiological pH, it exists predominantly as the negatively charged aspartate.
- Glutamic Acid (Glu, E): Carboxyl group; pKa around 4.3. At physiological pH, it exists predominantly as the negatively charged glutamate.
Polar Charged Amino Acids (Basic)
These amino acids have basic side chains with amino groups that are protonated at physiological pH.
- Lysine (Lys, K): ε-amino group; pKa around 10.5. At physiological pH, it carries a positive charge.
- Arginine (Arg, R): Guanidinium group; pKa around 12.5. At physiological pH, it carries a positive charge.
- Histidine (His, H): Imidazole group; pKa around 6.0. This is exceptionally important! Its pKa is close to physiological pH, meaning it can exist in both protonated and deprotonated forms at physiological pH. This makes histidine a crucial residue in many enzymatic active sites, facilitating proton transfer reactions and acting as a pH sensor.
Determining pKa Values
Several methods are employed to determine the pKa values of amino acid side chains:
- Titration: A classical method involving measuring pH changes as a strong base is added to a solution of the amino acid. The pKa is determined from the inflection point of the titration curve.
- Spectroscopy: Techniques like UV-Vis and NMR spectroscopy can be used to monitor the changes in the absorption or chemical shift of the side chain as a function of pH. These changes reflect the ionization state of the side chain and can be used to determine the pKa.
- Computational methods: Molecular dynamics simulations and quantum mechanical calculations can predict pKa values, particularly useful for estimating the effective pKa of side chains within proteins.
The Significance of pKa in Biological Systems
The pKa of amino acid side chains profoundly impacts several aspects of protein structure and function:
- Protein Folding: Electrostatic interactions between charged side chains influence protein folding and stability. The pKa values of these side chains dictate the strength and nature of these interactions.
- Enzyme Catalysis: Many enzymes utilize amino acid side chains with specific pKa values for catalytic activity. For example, the imidazole group of histidine, with its pKa near physiological pH, often participates in acid-base catalysis.
- Protein-ligand interactions: The pKa of side chains involved in binding sites can influence the affinity and specificity of protein-ligand interactions. Changes in the microenvironment of the binding site can alter the pKa of the residues, modulating the binding process.
- pH-dependent protein function: Some proteins exhibit pH-dependent activity due to the ionization state of specific side chains. This is particularly relevant in processes where pH gradients exist, such as across cell membranes.
- Post-translational modifications: Several post-translational modifications (PTMs) like phosphorylation alter the pKa of the modified residue, which consequently changes its interactions and function.
pKa Shifts in Proteins: The Microenvironment Matters
The pKa values discussed above are determined in aqueous solutions. However, within a protein, the microenvironment significantly alters the effective pKa of side chains. Several factors contribute to these pKa shifts:
- Hydrogen bonding: Hydrogen bonds can stabilize either the protonated or deprotonated form of a side chain, thus shifting its pKa.
- Electrostatic interactions: The presence of nearby charged residues can influence the pKa through electrostatic interactions. A negatively charged residue near an acidic side chain will raise its pKa, while a positively charged residue will lower it.
- Hydrophobic effects: Burial of a side chain within the protein's hydrophobic core can significantly impact its pKa. The reduced accessibility of water can affect the stability of the ionized form.
Conclusion: pKa – A Key to Understanding Proteins
The pKa of amino acid side chains is a fundamental parameter in understanding protein structure, function, and interactions. While the intrinsic pKa values provide a baseline, the effective pKa within the protein context is dynamically modulated by the microenvironment. This intricate interplay of factors highlights the complexity and sophistication of biological systems, emphasizing the importance of considering pKa shifts when studying protein behavior and function. Further research continues to unravel the intricacies of pKa values and their role in the biological world, opening doors to new therapeutic strategies and biotechnological advancements. Understanding these values is critical for numerous fields, including drug design, protein engineering, and the study of disease mechanisms. By grasping the concepts outlined in this guide, researchers and students can gain deeper insights into the fascinating world of proteins and their essential role in life.
Latest Posts
Latest Posts
-
Is Acid Catalyzed Hydration Syn Or Anti
Mar 27, 2025
-
1 F 1 S 1 S
Mar 27, 2025
-
What Is An Example Of A Statistical Question
Mar 27, 2025
-
What Group On The Periodic Table Is The Most Reactive
Mar 27, 2025
-
The Ph Of A Solution Is
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Pka Of Amino Acid Side Chains . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
