What Group On The Periodic Table Is The Most Reactive
Muz Play
Mar 27, 2025 · 6 min read
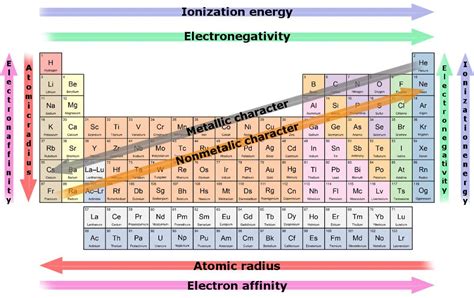
Table of Contents
What Group on the Periodic Table is the Most Reactive?
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. One of the most striking trends observed is the variation in reactivity across groups (vertical columns). While many factors influence reactivity, the question of which group is the most reactive often sparks debate. The answer, however, isn't a simple one, and requires understanding the nuances of chemical behavior. This article delves into the reactivity of different groups, ultimately providing a nuanced answer to this compelling question.
Understanding Chemical Reactivity
Before diving into specific groups, let's define chemical reactivity. Reactivity refers to the ease and speed with which an element undergoes a chemical reaction. Highly reactive elements readily form bonds with other elements or compounds, often with vigorous energy release (exothermic reactions). Conversely, unreactive elements, or noble gases, show little tendency to react.
Reactivity is primarily determined by several factors:
-
Electron Configuration: The arrangement of electrons in an atom's shells dictates its bonding behavior. Elements strive to achieve a stable electron configuration, often resembling that of noble gases (full outer shell). This drive towards stability is the fundamental driving force behind reactivity.
-
Electronegativity: This measures an atom's ability to attract electrons in a chemical bond. Highly electronegative elements readily attract electrons, making them reactive with electropositive elements (those readily losing electrons).
-
Ionization Energy: The energy required to remove an electron from an atom. Low ionization energy indicates high reactivity as the element readily loses electrons to form positive ions (cations).
-
Electron Affinity: The energy change when an atom gains an electron. High electron affinity implies a strong tendency to gain electrons, forming negative ions (anions) and thus high reactivity.
Group 1: The Alkali Metals – Highly Reactive Champions
The alkali metals (lithium, sodium, potassium, rubidium, cesium, and francium) are renowned for their exceptionally high reactivity. Located in Group 1, they possess a single valence electron in their outermost shell. This lone electron is easily lost, forming a +1 ion. This ease of electron loss translates to:
-
Vigorous Reactions with Water: Alkali metals react explosively with water, producing hydrogen gas and a metal hydroxide. The reaction intensity increases down the group, with cesium exhibiting the most dramatic reaction. The heat generated is often sufficient to ignite the hydrogen gas.
-
Reactions with Oxygen: Alkali metals readily react with oxygen in the air, forming oxides or peroxides. This necessitates their storage under inert conditions (e.g., oil) to prevent rapid oxidation.
-
Reactions with Halogens: They readily react with halogens (Group 17) to form ionic salts. These reactions are highly exothermic.
Why are they so reactive? Their low ionization energy and low electronegativity mean they readily lose their single valence electron to achieve a stable, noble gas configuration. The larger atomic size down the group further reduces ionization energy, amplifying reactivity.
Group 2: The Alkaline Earth Metals – Reactive, but Less So
Group 2 elements (beryllium, magnesium, calcium, strontium, barium, and radium) exhibit high reactivity, although less pronounced than alkali metals. They have two valence electrons, which are lost to form +2 ions. Their reactivity is still significant, although less dramatic than Group 1.
-
Reactions with Water: While less explosive than alkali metals, alkaline earth metals react with water, producing hydrogen gas and a metal hydroxide. The reactivity increases down the group, with beryllium being relatively unreactive and barium reacting vigorously.
-
Reactions with Oxygen: They react with oxygen to form oxides, albeit less readily than alkali metals.
-
Reactions with Halogens: Like alkali metals, they readily react with halogens to form ionic salts.
Group 17: The Halogens – Highly Reactive Non-metals
The halogens (fluorine, chlorine, bromine, iodine, and astatine) constitute another group of highly reactive elements, but unlike Groups 1 and 2, their reactivity stems from their tendency to gain electrons. They have seven valence electrons, needing only one more to achieve a stable octet.
-
Reactions with Metals: Halogens readily react with metals, forming ionic salts. Fluorine, being the most electronegative element, shows the highest reactivity.
-
Reactions with Hydrogen: They react with hydrogen to form hydrogen halides (HF, HCl, HBr, HI), which are highly acidic.
-
Reactions with Other Non-metals: Halogens can also react with other non-metals, often forming covalent compounds.
Why are they so reactive? Their high electronegativity and high electron affinity mean they readily gain electrons to achieve a stable configuration. Fluorine, with the highest electronegativity, is the most reactive halogen.
Comparing Reactivity Across Groups
While Groups 1 and 17 both demonstrate high reactivity, the type of reactivity differs significantly. Alkali metals readily lose electrons, while halogens readily gain electrons. This difference highlights the importance of considering the specific mechanism of reactivity.
The alkaline earth metals (Group 2) show reactivity intermediate between alkali metals and other groups. Their two valence electrons are more difficult to remove compared to the single electron in alkali metals, resulting in lower reactivity.
The Noble Gases: The Unreactive Exception
Group 18, the noble gases (helium, neon, argon, krypton, xenon, and radon), are remarkably unreactive. They possess a full outer electron shell (octet), making them exceptionally stable. This stable configuration minimizes their tendency to participate in chemical reactions.
Although historically considered completely inert, heavier noble gases (like xenon and radon) have been shown to form compounds under specific conditions, demonstrating that even the "unreactive" can react under extreme circumstances. However, their overall unreactivity remains a defining characteristic.
Conclusion: A Nuance to the "Most Reactive" Question
There's no single definitive answer to the question of "which group is the most reactive." The answer depends on the context and the definition of "reactive."
-
Considering the intensity and ease of reactions: Alkali metals (Group 1) exhibit the most dramatic and readily observable reactions, particularly with water. Their reactions are often violent and exothermic.
-
Considering electron affinity and electronegativity: Halogens (Group 17) display an unparalleled ability to attract and gain electrons, leading to highly energetic reactions with a wide range of elements.
Ultimately, both Groups 1 and 17 showcase exceptional reactivity, albeit through different mechanisms. The alkaline earth metals (Group 2) show significant reactivity but are less dramatic than Groups 1 and 17. The noble gases (Group 18) stand apart, showcasing exceptional stability and minimal reactivity. The "most reactive" title depends heavily on the criteria used for judging reactivity. The extreme reactivity of Group 1 is visually striking, while the high electronegativity and electron affinity of Group 17 makes it a powerful oxidizing agent. Understanding the nuances of reactivity in different groups enriches our understanding of the periodic table and the fascinating world of chemical reactions.
Latest Posts
Latest Posts
-
Electrons In The Outermost Energy Level Are Called
Mar 30, 2025
-
Definition For Subtraction Property Of Equality
Mar 30, 2025
-
Why Is Rusting A Chemical Change
Mar 30, 2025
-
Difference Between Dipole Dipole And London Dispersion Forces
Mar 30, 2025
-
Which Is The Most Reactive Element
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Group On The Periodic Table Is The Most Reactive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
