Two Different Isotopes Of An Element Have Different
Muz Play
Mar 25, 2025 · 6 min read
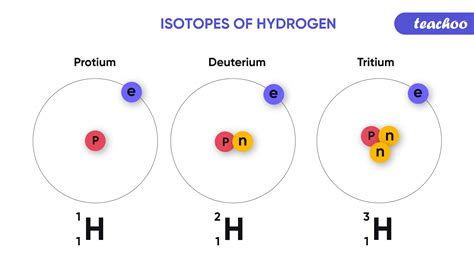
Table of Contents
- Two Different Isotopes Of An Element Have Different
- Table of Contents
- Two Different Isotopes of an Element Have Different... Properties!
- Understanding Isotopes: A Foundation
- Key Differences Between Isotopes: A Detailed Exploration
- 1. Mass Number and Atomic Mass
- 2. Nuclear Stability and Radioactivity
- 3. Physical Properties: Density and Melting/Boiling Points
- 4. Chemical Properties: Isotopic Effects
- 5. Applications: A Wide Spectrum
- Isotope Separation: A Technological Challenge
- Conclusion: The Significance of Isotopic Variations
- Latest Posts
- Latest Posts
- Related Post
Two Different Isotopes of an Element Have Different... Properties!
Isotopes, those fascinating variations of the same element, are often misunderstood. While sharing the same atomic number (number of protons), they differ in their number of neutrons. This seemingly subtle difference leads to a surprisingly wide array of variations in their properties, impacting everything from their stability to their applications in various fields. Let's delve into the fascinating world of isotopes and explore the key differences between them.
Understanding Isotopes: A Foundation
Before we delve into the specifics of isotopic differences, let's establish a solid understanding of what isotopes actually are. An element is defined by the number of protons in its nucleus – this is its atomic number. However, the number of neutrons can vary. These variations, with the same number of protons but different numbers of neutrons, are called isotopes.
For example, consider carbon (atomic number 6). The most common isotope is Carbon-12 (¹²C), with 6 protons and 6 neutrons. However, there's also Carbon-13 (¹³C) with 6 protons and 7 neutrons, and Carbon-14 (¹⁴C) with 6 protons and 8 neutrons. These are all isotopes of carbon.
The number after the element's name represents the mass number, which is the sum of protons and neutrons. This is crucial because it directly influences several key properties.
Key Differences Between Isotopes: A Detailed Exploration
The differences between isotopes manifest in various ways. Let's explore some of the most significant:
1. Mass Number and Atomic Mass
The most obvious difference is the mass number. This directly affects the atomic mass, which is the weighted average of the masses of all the isotopes of an element, taking into account their relative abundance. Heavier isotopes contribute more to the atomic mass than lighter ones. This difference in mass has far-reaching consequences.
For example, the difference in mass between isotopes of uranium (like Uranium-235 and Uranium-238) is crucial for nuclear fission. The slightly lighter Uranium-235 is far more likely to undergo fission when bombarded with neutrons compared to Uranium-238.
2. Nuclear Stability and Radioactivity
This is perhaps the most dramatic difference between isotopes. Some isotopes are stable, meaning their nuclei remain intact indefinitely. Others are unstable or radioactive, meaning their nuclei decay spontaneously over time, emitting particles or energy in the process.
Radioactive decay can occur through various mechanisms, including alpha decay, beta decay, and gamma decay. The rate of decay is characterized by the half-life, which is the time it takes for half of the radioactive atoms in a sample to decay. Half-lives range from fractions of a second to billions of years, depending on the isotope.
Carbon-14, with its relatively long half-life of approximately 5,730 years, is famously used in radiocarbon dating to determine the age of organic materials. The different decay rates of isotopes are exploited in various applications, including medical imaging and cancer treatment.
3. Physical Properties: Density and Melting/Boiling Points
While the number of protons largely determines the chemical properties of an element, the number of neutrons subtly influences some physical properties. The mass difference between isotopes can lead to slightly different densities. Although the effect is often minor, it can be measurable and relevant in certain contexts, especially in isotopic separation techniques.
Furthermore, the differences in intermolecular forces due to the mass variation can lead to small differences in melting and boiling points, although these are generally insignificant compared to differences between different elements. However, in highly sensitive measurements or specialized applications, these subtle variations can become relevant.
4. Chemical Properties: Isotopic Effects
While chemical properties are primarily determined by the number of electrons (which is equal to the number of protons), there are subtle isotopic effects that can arise due to the difference in mass between isotopes. These effects are usually small but can be significant in certain circumstances, primarily in situations where the mass difference is a substantial fraction of the total mass of the molecule.
These effects manifest in various ways, including differences in reaction rates (kinetic isotope effects), equilibrium constants (equilibrium isotope effects), and even changes in molecular vibrations and spectroscopy. Heavy isotopes tend to react slightly slower than their lighter counterparts, which is exploited in certain chemical studies and applications.
5. Applications: A Wide Spectrum
The differences between isotopes lead to a wide array of applications across various fields:
-
Nuclear Medicine: Radioactive isotopes are used in diagnostic imaging (like PET scans) and radiotherapy to treat cancer. The radioactive decay emits particles or energy that can be detected, allowing doctors to image organs or target cancerous cells.
-
Nuclear Power: Isotopes like Uranium-235 are used as fuel in nuclear reactors. The controlled fission of these isotopes releases immense energy.
-
Radiocarbon Dating: Carbon-14 is used to determine the age of organic materials, providing valuable insights into archaeology and paleontology.
-
Industrial Applications: Isotopes are used as tracers in various industrial processes, such as tracking the movement of materials or analyzing the efficiency of chemical reactions.
-
Scientific Research: Isotopes are invaluable tools in scientific research, enabling scientists to study various processes in chemistry, biology, and environmental science. Stable isotopes are frequently used in metabolic studies to trace the fate of nutrients and metabolites within a living organism.
Isotope Separation: A Technological Challenge
Separating isotopes is a significant technological challenge because they possess almost identical chemical properties. Several techniques are employed, each suited to specific isotopes and quantities:
-
Gaseous Diffusion: This method exploits the slight difference in diffusion rates of gaseous isotopes. Lighter isotopes diffuse faster than heavier ones.
-
Centrifugation: This technique uses centrifugal force to separate isotopes based on their mass difference. Heavier isotopes tend to concentrate towards the outside of the centrifuge.
-
Laser Isotope Separation: This sophisticated method uses lasers to selectively excite and ionize specific isotopes, allowing for their separation.
-
Chromatography: Different forms of chromatography, including gas chromatography and liquid chromatography, can be used for isotope separation under certain circumstances, particularly when coupled with mass spectrometry for detection.
Conclusion: The Significance of Isotopic Variations
The differences between isotopes of the same element, while often subtle, have profound implications across diverse scientific disciplines and technological applications. Their distinct mass numbers, nuclear stability, and resulting properties make them invaluable tools in fields ranging from medicine and energy production to archaeology and environmental science. Understanding the nuances of isotopic variations is crucial for advancing our knowledge and utilizing these differences for human benefit. The continuous development of isotope separation technologies further enhances the accessibility and applicability of these remarkable variations of elements. From radiocarbon dating artifacts to powering nuclear reactors, the world around us depends on the properties of specific isotopes, showcasing their profound influence on our lives.
Latest Posts
Latest Posts
-
Lewis Acid And Base Practice Problems
Mar 28, 2025
-
During Which Phase Of Meiosis Do Homologous Chromosomes Separate
Mar 28, 2025
-
Relation Between Electric Field And Electric Potential
Mar 28, 2025
-
Finding The Standard Matrix Of A Linear Transformation
Mar 28, 2025
-
What Is A Quarter In Percentage
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Two Different Isotopes Of An Element Have Different . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
