Lock And Key Model Of Enzymes
Muz Play
Mar 18, 2025 · 7 min read
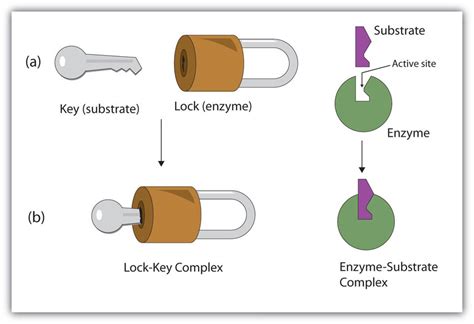
Table of Contents
The Lock and Key Model of Enzymes: A Comprehensive Overview
The lock and key model, a cornerstone of biochemistry, provides a simplified yet insightful explanation of enzyme-substrate interactions. While advancements have revealed its limitations, understanding this model remains crucial for grasping fundamental enzymatic principles. This article delves into the intricacies of the lock and key model, exploring its strengths, weaknesses, and its place within the broader context of enzyme kinetics and catalysis.
Understanding Enzymes and Their Function
Before diving into the specifics of the lock and key model, let's establish a firm understanding of enzymes themselves. Enzymes are biological catalysts, primarily proteins, that significantly accelerate the rate of biochemical reactions within living organisms. They achieve this acceleration without being consumed in the process. Their remarkable catalytic power stems from their unique three-dimensional structures, which create specific binding sites for their target molecules, known as substrates.
The Importance of Enzyme Specificity
Enzyme specificity is a hallmark feature, ensuring that each enzyme acts upon a particular substrate or a small group of closely related substrates. This specificity arises from the precise arrangement of amino acid residues within the enzyme's active site, a region designed to bind the substrate and facilitate the catalytic process. This highly selective interaction is crucial for maintaining the intricate balance of metabolic pathways within cells.
The Lock and Key Model: A Simple Analogy
The lock and key model, proposed by Emil Fischer in 1894, uses a simple analogy to illustrate enzyme-substrate interaction. It posits that the enzyme's active site (the "lock") possesses a rigid, pre-formed structure perfectly complementary to the shape of the substrate (the "key"). Only the correct substrate, with the precise shape and chemical properties, can fit into the active site. This "perfect fit" initiates the catalytic process, leading to the formation of products.
Visualizing the Interaction
Imagine a key, intricately shaped to fit a specific lock. Only that particular key can unlock the door. Similarly, only the specific substrate with the complementary shape and chemical groups can bind to the enzyme's active site. This binding induces a conformational change, often subtle, within the enzyme, further optimizing the interaction and facilitating catalysis.
Strengths of the Lock and Key Model
The lock and key model, despite its simplicity, offers several strengths:
-
Intuitive Understanding: It provides an easily understandable and memorable analogy for explaining enzyme specificity and substrate binding. Its simplicity makes it an excellent teaching tool, particularly at introductory levels.
-
Explains Specificity: The model elegantly explains the high degree of specificity observed in enzymatic reactions. Only substrates with the correct shape and chemical properties can bind effectively.
-
Foundation for Further Models: Although superseded by more refined models, the lock and key model serves as a crucial foundation for understanding the more complex induced fit model. It provides a basic framework upon which more sophisticated theories are built.
Limitations of the Lock and Key Model
While the lock and key model offers a valuable introductory understanding, it suffers from several significant limitations:
-
Rigid Active Site: The model assumes a completely rigid and unchanging active site. This is an oversimplification, as enzyme active sites are often flexible and dynamic, undergoing conformational changes upon substrate binding.
-
Fails to Explain Enzyme Regulation: The model doesn't adequately explain the mechanisms of enzyme regulation, such as allosteric regulation or feedback inhibition, which involve conformational changes influencing substrate binding.
-
Ignores Transition State Stabilization: The lock and key model doesn't explain how enzymes stabilize the transition state of the reaction, a crucial aspect of enzymatic catalysis. The actual catalytic mechanism involves more than just a simple fit.
The Induced Fit Model: A Refinement of the Lock and Key Model
Daniel Koshland's induced fit model, proposed in 1958, addresses the limitations of the lock and key model. This model suggests that the enzyme's active site is not a rigid, pre-formed structure but rather a flexible one that undergoes conformational changes upon substrate binding. The binding of the substrate induces a change in the enzyme's shape, optimizing the interaction and facilitating catalysis.
A More Dynamic View of Enzyme-Substrate Interaction
In the induced fit model, the initial interaction between the enzyme and substrate may be weak. However, this weak binding triggers conformational changes in the enzyme, leading to a tighter, more complementary fit. This induced fit optimizes the positioning of catalytic residues within the active site, enhancing the efficiency of catalysis. It's a dynamic process, not a static "lock and key" interaction.
Comparing the Lock and Key and Induced Fit Models
| Feature | Lock and Key Model | Induced Fit Model |
|---|---|---|
| Active Site | Rigid, pre-formed structure | Flexible, undergoes conformational changes upon binding |
| Substrate Binding | Strict complementarity | Initial weak binding followed by conformational changes |
| Catalysis | Simple binding initiates catalysis | Induced fit optimizes catalysis |
| Regulation | Not adequately explained | Explains allosteric regulation and feedback inhibition |
| Transition State | Not explicitly addressed | Explains stabilization of the transition state |
The Role of Non-Covalent Interactions in Enzyme-Substrate Binding
The binding of the substrate to the enzyme's active site is primarily driven by a variety of weak, non-covalent interactions. These interactions, including hydrogen bonds, ionic interactions, van der Waals forces, and hydrophobic interactions, collectively contribute to the overall binding affinity. The strength and specificity of these interactions dictate the selectivity and efficiency of the enzyme.
The Importance of Weak Interactions
The transient nature of weak interactions is crucial. They allow the enzyme to bind the substrate reversibly, ensuring that the product can be released once the reaction is complete. The cumulative effect of numerous weak interactions provides significant binding energy, ensuring strong and specific substrate binding.
Enzyme Kinetics and the Lock and Key Model
Enzyme kinetics studies the rates of enzyme-catalyzed reactions. The Michaelis-Menten equation, a fundamental equation in enzyme kinetics, describes the relationship between the reaction rate and the substrate concentration. While the Michaelis-Menten equation doesn't explicitly incorporate the lock and key model, it is implicitly based on the assumption of a specific enzyme-substrate interaction. The equation's parameters, such as K<sub>m</sub> (Michaelis constant) and V<sub>max</sub> (maximum reaction velocity), reflect the affinity and catalytic efficiency of the enzyme.
Beyond the Lock and Key Model: Advances in Understanding Enzyme Mechanism
While the lock and key and induced fit models provide valuable conceptual frameworks, modern research has revealed the intricacies of enzyme catalysis are far more complex. Advanced techniques such as X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and molecular dynamics simulations have provided detailed insights into the dynamic nature of enzyme-substrate interactions and the mechanisms of catalysis.
The Importance of Transition State Stabilization
One crucial aspect not fully captured by the simpler models is the enzyme's role in stabilizing the transition state of the reaction. The transition state is a high-energy intermediate state that must be overcome for the reaction to proceed. Enzymes achieve this stabilization through precise interactions with the substrate, reducing the activation energy and thus accelerating the reaction rate.
Conclusion: The Enduring Legacy of the Lock and Key Model
The lock and key model, while a simplification, remains a valuable tool for understanding basic enzyme-substrate interactions. Its strengths lie in its simplicity and intuitive explanation of enzyme specificity. However, its limitations highlight the need for more sophisticated models, such as the induced fit model, which better capture the dynamic nature of enzyme function. Advances in research continue to refine our understanding of enzyme mechanisms, but the fundamental principles encapsulated in the lock and key model provide a solid foundation for this ongoing exploration. Future research will undoubtedly continue to build upon these foundational models, further illuminating the complex and fascinating world of enzymatic catalysis. This deeper understanding has far-reaching implications in various fields, including drug design, biotechnology, and metabolic engineering. The ongoing exploration of enzyme mechanisms promises to reveal even more intricate details and ultimately contribute to advancements in numerous scientific disciplines.
Latest Posts
Latest Posts
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
-
Difference Between A Strong And Weak Acid
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Lock And Key Model Of Enzymes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
