Mass Of A Single Hydrogen Atom
Muz Play
Mar 15, 2025 · 5 min read
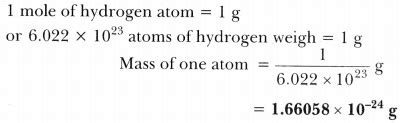
Table of Contents
The Mass of a Single Hydrogen Atom: A Deep Dive
The seemingly simple question, "What is the mass of a single hydrogen atom?" opens a fascinating window into the world of atomic physics, chemistry, and measurement. While the answer might seem straightforward at first glance, a deeper exploration reveals layers of complexity and nuance related to isotopes, measurement techniques, and the very nature of atomic mass. This article will delve into these intricacies, providing a comprehensive understanding of the mass of a single hydrogen atom and its implications.
Understanding Atomic Mass and Isotopes
Before diving into the specifics of hydrogen's mass, it's crucial to understand the concepts of atomic mass and isotopes. Atomic mass, also known as atomic weight, is the mass of an atom, typically expressed in atomic mass units (amu) or daltons (Da). One amu is defined as 1/12 the mass of a carbon-12 atom.
Hydrogen, the simplest element, is unique in that it possesses three naturally occurring isotopes: protium (¹H), deuterium (²H or D), and tritium (³H or T). Isotopes are atoms of the same element with the same number of protons but differing numbers of neutrons. This difference in neutron count directly affects the atom's mass.
-
Protium (¹H): This is the most abundant isotope of hydrogen, comprising over 99.98% of naturally occurring hydrogen. It consists of one proton and no neutrons.
-
Deuterium (²H or D): Deuterium contains one proton and one neutron, making it approximately twice as massive as protium. It's a stable isotope, meaning it doesn't undergo radioactive decay.
-
Tritium (³H or T): Tritium possesses one proton and two neutrons. It's a radioactive isotope, undergoing beta decay with a half-life of approximately 12.3 years.
Determining the Mass of a Single Hydrogen Atom: The Challenges
Determining the mass of a single hydrogen atom isn't a simple matter of weighing it on a scale. Atoms are incredibly small, far too tiny to be measured directly using conventional methods. Instead, scientists rely on sophisticated techniques based on mass spectrometry and other advanced analytical methods.
Mass Spectrometry: A Powerful Tool
Mass spectrometry is a fundamental technique used to determine the mass-to-charge ratio of ions. In the context of measuring the mass of a hydrogen atom, a sample of hydrogen gas is ionized, creating charged hydrogen atoms (H⁺). These ions are then accelerated through a magnetic field, which separates them based on their mass-to-charge ratio. By carefully analyzing the resulting spectrum, scientists can accurately determine the mass of each hydrogen isotope.
High Precision Measurements
The accuracy of mass measurements is crucial in this context. Advanced mass spectrometry techniques, coupled with careful calibration and data analysis, allow for extremely precise determination of atomic masses. The masses of the hydrogen isotopes are known with extraordinary precision, allowing for accurate calculations related to various scientific applications.
The Masses of Hydrogen Isotopes: Precise Values
Based on these advanced techniques, the following highly precise mass values have been determined for the hydrogen isotopes:
-
Protium (¹H): 1.00782503223 amu
-
Deuterium (²H): 2.01410177812 amu
-
Tritium (³H): 3.0160492777 amu
Calculating the Average Atomic Mass of Hydrogen
The average atomic mass of hydrogen, often found on periodic tables, represents the weighted average of the masses of its naturally occurring isotopes. This weighted average takes into account the relative abundance of each isotope. Given the overwhelming abundance of protium, the average atomic mass of hydrogen is very close to the mass of a protium atom. However, the small but significant contributions of deuterium and tritium must be included for accuracy.
The calculation involves multiplying the mass of each isotope by its natural abundance and summing the results:
Average atomic mass of hydrogen ≈ (abundance of ¹H × mass of ¹H) + (abundance of ²H × mass of ²H) + (abundance of ³H × mass of ³H)
The exact value obtained will depend on the specific abundances used, as these can vary slightly depending on the source of the hydrogen sample. However, the value typically cited is approximately 1.00794 amu.
Implications of the Mass of a Single Hydrogen Atom
The precisely determined mass of a single hydrogen atom and its isotopes has profound implications across various scientific fields:
Nuclear Physics and Astrophysics:
Understanding the mass of hydrogen isotopes is critical for nuclear physics research, particularly in the study of nuclear fusion reactions which power stars. The mass difference between reactants and products in fusion processes is directly related to the energy released, a concept encapsulated in Einstein's famous equation, E=mc². Accurate knowledge of atomic masses is fundamental to modeling these energy-releasing reactions.
Chemistry and Chemical Calculations:
Accurate atomic masses are crucial for precise chemical calculations, such as determining the molar mass of compounds, calculating stoichiometric ratios in chemical reactions, and understanding the behavior of molecules and chemical systems.
Mass Spectrometry and Analytical Chemistry:
The mass of a hydrogen atom serves as a fundamental reference point in mass spectrometry. Accurate measurements of the masses of other ions are often compared to known hydrogen isotope masses for calibration and analysis.
Environmental Science and Isotope Geochemistry:
The relative abundances of deuterium and tritium in water samples can be used to trace the origin and movement of water through the environment. This technique is employed in hydrological studies and climate research.
Medical Applications and Nuclear Medicine:
Tritium, with its radioactive properties, finds applications in medical imaging and research. Its specific radioactive decay characteristics are closely linked to its mass and nuclear structure.
Conclusion
The mass of a single hydrogen atom, while seemingly a simple concept, encompasses a wealth of scientific knowledge and sophisticated measurement techniques. The precise determination of the masses of its isotopes—protium, deuterium, and tritium—has widespread implications across numerous fields, from nuclear physics and astrophysics to chemistry and environmental science. The ongoing refinement of these mass measurements continues to drive advancements in our understanding of the fundamental building blocks of matter and the universe itself. This detailed exploration highlights the importance of accurate measurements at the atomic level and their critical role in expanding our scientific knowledge.
Latest Posts
Latest Posts
-
Que Es La Descomposicion De Acidos
Mar 15, 2025
-
Which Factor Affects Congressional Approval Ratings The Most
Mar 15, 2025
-
Fourier Transform Of A Differential Equation
Mar 15, 2025
-
Which One Neutral Charge Proton Or Neutron
Mar 15, 2025
-
A Solids Volume And Shape Is Defintie
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Mass Of A Single Hydrogen Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
