Melting Point Of Water In Kelvin
Muz Play
Mar 23, 2025 · 6 min read
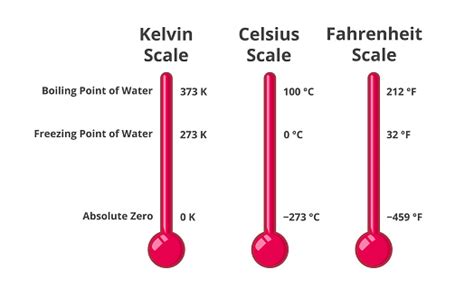
Table of Contents
The Melting Point of Water in Kelvin: A Deep Dive
The melting point of water, a seemingly simple concept, holds significant importance across various scientific disciplines and everyday life. Understanding its value in Kelvin, the absolute temperature scale, provides a crucial foundation for numerous applications, from understanding phase transitions to designing industrial processes. This article delves deep into the melting point of water in Kelvin, exploring its precise value, the factors influencing it, and its broader implications.
Defining the Melting Point
Before exploring the Kelvin value, let's establish a clear definition. The melting point of a substance is the temperature at which it transitions from a solid state (ice, in the case of water) to a liquid state (water). This transition occurs at a specific temperature under standard pressure conditions. Crucially, this is an equilibrium point; at the melting point, solid and liquid phases coexist in thermodynamic equilibrium.
The Melting Point of Water: Celsius and Kelvin
While commonly expressed in degrees Celsius (°C), the melting point of water is precisely 0°C at standard atmospheric pressure (1 atmosphere or 101.325 kPa). However, for many scientific applications, the Kelvin scale (K) is preferred. The Kelvin scale is an absolute temperature scale, meaning its zero point (0 K) represents absolute zero – the theoretical lowest possible temperature where all molecular motion ceases.
The conversion between Celsius and Kelvin is straightforward:
K = °C + 273.15
Therefore, the melting point of water in Kelvin is:
0°C + 273.15 = 273.15 K
This value, 273.15 K, represents the temperature at which ice transforms into liquid water under standard atmospheric pressure. This seemingly simple number underpins a vast array of scientific calculations and practical applications.
Factors Affecting the Melting Point of Water
While 273.15 K is the standard melting point, several factors can influence the precise temperature at which water melts. These factors primarily relate to changes in pressure and the presence of impurities.
Pressure's Influence
Pressure significantly impacts the melting point of water. Unlike most substances, water exhibits an anomalous behavior: its melting point decreases with increasing pressure. This is due to the unique structure of ice, where the hydrogen bonds create a less dense structure compared to liquid water. Applying pressure forces the water molecules closer together, favoring the denser liquid state and thus lowering the melting point. This phenomenon is crucial in various geological processes, including glacier movement.
At very high pressures, the melting point of ice can be significantly lower than 273.15 K. This is why ice skates can glide smoothly on ice; the pressure from the skates locally lowers the melting point of the ice, creating a thin layer of water that reduces friction.
Impact of Impurities
The presence of impurities in water, such as dissolved salts or other substances, also affects its melting point. These impurities disrupt the hydrogen bonding network in ice, making it easier for the ice to melt. This phenomenon is known as freezing point depression and is utilized in various applications, such as de-icing roads with salt. The addition of salt lowers the freezing point (and thus the melting point) of water, preventing ice formation at temperatures slightly below 0°C.
The magnitude of the freezing point depression depends on the concentration of the solute (impurity). A higher concentration leads to a greater depression of the melting point. This principle is fundamental to various chemical and physical processes.
Applications of Water's Melting Point
The knowledge of water's melting point in Kelvin, and the factors influencing it, has profound implications across diverse fields:
Meteorology and Climatology
Understanding the melting point of water is crucial for meteorological and climatological studies. Predicting weather patterns, analyzing climate change impacts, and modeling glacial melt all rely on accurate knowledge of the melting point and its sensitivity to temperature and pressure fluctuations. The phase transitions of water play a pivotal role in atmospheric processes and global climate dynamics.
Biology and Chemistry
In biological systems, the melting point of water is fundamental to life itself. The properties of water, its high heat capacity, and its melting point ensure a stable environment for biological processes. In chemistry, the melting point is a crucial physical property used for identifying and characterizing substances. The precise determination of melting points aids in substance purification and analysis.
Engineering and Industry
In various industrial processes, controlling the temperature around the melting point of water is crucial. Examples include:
- Food processing: Freezing and thawing food products require precise temperature control to maintain quality and prevent spoilage.
- HVAC systems: Heating, ventilation, and air conditioning systems rely on the properties of water, including its melting point, for efficient heat transfer.
- Material science: Understanding the effects of pressure and impurities on the melting point of water is vital in designing and synthesizing materials with specific properties.
Everyday Life
Even in everyday life, the melting point of water plays a vital role. From understanding why ice melts in warm temperatures to using ice for cooling beverages, the knowledge of water's melting point is implicitly embedded in our daily routines.
Advanced Concepts and Research
Research continues to explore the finer details of water's melting point, particularly under extreme conditions and within confined spaces (e.g., nano-confined water). Studies examining the behavior of water at interfaces and the influence of various factors on the melting point contribute to a deeper understanding of this fundamental property.
Isotopic Effects
Even the isotopic composition of water influences its melting point. Water molecules composed of heavier isotopes (deuterium instead of hydrogen) have slightly different melting points compared to regular water. These subtle differences have implications in various scientific areas, including hydrology and climate science.
Supercooling
Water can exist in a liquid state below its normal freezing point (supercooling). This phenomenon occurs when the water is extremely pure and lacks nucleation sites for ice crystal formation. Supercooled water can exist in a metastable state until a disturbance triggers its rapid freezing. This is relevant to various scientific and atmospheric processes.
Conclusion
The melting point of water at 273.15 K is more than just a number; it's a fundamental constant that underpins countless natural phenomena and technological applications. Understanding this value, its sensitivity to pressure and impurities, and its broader implications is critical for advancements in various scientific and engineering disciplines. Continued research into the intricacies of water's phase transitions promises to unveil further insights into this essential aspect of our world. From the macroscopic scale of glaciers to the microscopic scale of biological processes, the melting point of water continues to be a subject of profound scientific interest and practical significance. Its simple numerical representation belies its profound impact on our understanding of the natural world and our technological capabilities.
Latest Posts
Latest Posts
-
Is Vegetable Oil A Pure Substance Or A Mixture
Mar 24, 2025
-
Is Tap Water Homogeneous Or Heterogeneous
Mar 24, 2025
-
What Is The Electron Configuration For Boron
Mar 24, 2025
-
The Fourth Ventricle Is Represented By Letter
Mar 24, 2025
-
How To Read Limits On A Graph
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Melting Point Of Water In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
