Normal Phase Vs Reverse Phase Hplc
Muz Play
Mar 22, 2025 · 6 min read
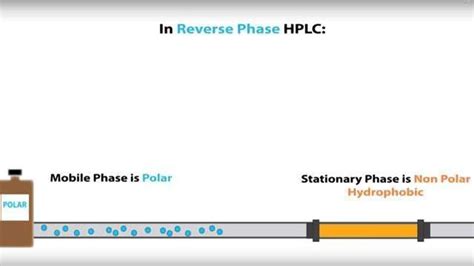
Table of Contents
Normal Phase vs. Reverse Phase HPLC: A Comprehensive Guide
High-Performance Liquid Chromatography (HPLC) is a powerful analytical technique used to separate, identify, and quantify components in a mixture. A crucial aspect of HPLC is the choice between normal phase and reverse phase chromatography, each offering unique advantages and disadvantages depending on the sample and the desired separation. This comprehensive guide delves into the intricacies of normal phase and reverse phase HPLC, comparing and contrasting their principles, applications, and suitability for various analytical needs.
Understanding the Fundamentals: Stationary and Mobile Phases
Both normal phase and reverse phase HPLC rely on the interaction between the analyte (the substance being analyzed) and the stationary and mobile phases. The stationary phase is a solid material packed into a column, while the mobile phase is a liquid solvent that carries the analyte through the column. The separation occurs based on the differential affinities of the analytes for these two phases.
Normal Phase HPLC
In normal phase HPLC, the stationary phase is polar and the mobile phase is nonpolar. Common stationary phases include silica gel, alumina, and amino-bonded silica. The mobile phase typically consists of solvents like hexane, heptane, or dichloromethane, potentially with small percentages of more polar modifiers like alcohols or ethyl acetate.
The separation mechanism in normal phase chromatography is based on the adsorption of analytes onto the polar stationary phase. More polar analytes interact more strongly with the stationary phase and therefore elute later, while less polar analytes elute faster.
Reverse Phase HPLC
Reverse phase HPLC is the most commonly used HPLC technique. It employs a nonpolar stationary phase and a polar mobile phase. The most common stationary phase is C18-bonded silica, which has octadecyl (C18) hydrocarbon chains grafted onto the silica surface. Other common phases include C8, phenyl, and cyano. The mobile phase is typically a mixture of water and an organic solvent such as acetonitrile or methanol.
In reverse phase chromatography, the separation is based on the partitioning of analytes between the nonpolar stationary phase and the polar mobile phase. Nonpolar analytes interact more strongly with the stationary phase and elute later, while polar analytes interact more strongly with the mobile phase and elute faster.
Key Differences: A Comparative Analysis
The table below summarizes the key differences between normal phase and reverse phase HPLC:
| Feature | Normal Phase HPLC | Reverse Phase HPLC |
|---|---|---|
| Stationary Phase | Polar (e.g., silica gel) | Nonpolar (e.g., C18-bonded silica) |
| Mobile Phase | Nonpolar (e.g., hexane) | Polar (e.g., water/acetonitrile) |
| Separation | Adsorption | Partitioning |
| Polarity | Polar compounds elute last | Polar compounds elute first |
| Solvent Strength | Increases with polarity | Increases with organic solvent content |
| Sensitivity | Can be affected by water | Less sensitive to water |
| Applications | Sugars, amino acids, isomers | Most applications, proteins, peptides |
Advantages and Disadvantages
Both techniques possess unique strengths and limitations:
Normal Phase HPLC: Advantages and Disadvantages
Advantages:
- Excellent for separating polar compounds: Normal phase HPLC is particularly effective in separating closely related polar compounds that are difficult to resolve using reverse phase methods.
- High selectivity for isomers: The strong interactions in normal phase can lead to excellent resolution of isomers, based on subtle differences in their structure.
- Compatibility with certain detectors: Normal phase is sometimes preferred for certain detectors, especially those less compatible with high water contents.
Disadvantages:
- Lower reproducibility: The stationary phase can be more sensitive to moisture, potentially impacting reproducibility.
- Limited solvent choices: The range of solvents suitable for normal phase is often narrower than that for reverse phase.
- Peak tailing: This is a common problem that can affect quantitation.
Reverse Phase HPLC: Advantages and Disadvantages
Advantages:
- High reproducibility: C18 columns are relatively stable and provide excellent reproducibility.
- Wide range of solvent choices: A broader range of solvents can be used, providing greater flexibility in method development.
- High efficiency: Reverse phase separations often achieve higher efficiency compared to normal phase.
- Ease of use: The technique is generally easier to set up and operate.
- Wide Applicability: It is the most widely used technique applicable to a broader range of compounds.
Disadvantages:
- Less effective for highly polar compounds: The limited interaction of highly polar compounds with the nonpolar stationary phase can hinder separation.
- Sensitivity to ionic strength: High ionic strength in the mobile phase can affect retention and peak shape.
Method Development Considerations
Choosing the appropriate HPLC method requires careful consideration of several factors:
- Analyte properties: The polarity and other physicochemical properties of the analytes are crucial in determining the best approach.
- Sample matrix: The nature of the sample matrix can influence the choice of mobile phase and column.
- Desired resolution: The desired separation efficiency and resolution will dictate the method parameters.
- Detector compatibility: The choice of detector needs to be compatible with the mobile phase.
Applications of Normal Phase and Reverse Phase HPLC
Normal Phase HPLC Applications
Normal phase HPLC finds application in several fields, including:
- Separation of sugars and carbohydrates: The strong interactions between polar sugars and the stationary phase allow for excellent separation of these closely related compounds.
- Analysis of chiral compounds: Normal phase can achieve high enantiomeric resolution, particularly with chiral stationary phases.
- Separation of lipids: Normal phase is sometimes used for the analysis of lipids, although this is less common than in reverse phase.
- Analysis of some pharmaceuticals: Certain pharmaceuticals with polar functionalities may be more effectively separated using normal phase.
- Purification of natural products: The technique is used for isolating and purifying natural products from complex mixtures.
Reverse Phase HPLC Applications
Reverse phase HPLC is the workhorse of HPLC, used across various scientific disciplines, including:
- Pharmaceutical analysis: It's widely used for the analysis of pharmaceuticals, including drug purity testing and quantification.
- Biopharmaceutical analysis: Reverse phase is indispensable for analyzing proteins, peptides, and other biomolecules.
- Environmental monitoring: It's used to analyze environmental pollutants such as pesticides and herbicides.
- Food analysis: Used for analyzing components in food and beverages, like vitamins and antioxidants.
- Forensic science: It plays a role in forensic investigations through the analysis of various substances.
- Clinical chemistry: Essential in analyzing biological fluids for diagnostics.
Conclusion: Choosing the Right Technique
The selection between normal phase and reverse phase HPLC hinges on the specific analytical needs. Reverse phase HPLC, with its widespread applicability, reproducibility, and ease of use, remains the dominant technique. However, normal phase HPLC offers distinct advantages in certain situations, particularly for separating polar compounds and isomers with high selectivity. A thorough understanding of both techniques and careful method development are critical for achieving optimal results in HPLC analysis. By considering the properties of the analytes, the sample matrix, and the available detectors, researchers can select the most appropriate technique and optimize the separation to meet their analytical goals. The choice ultimately depends on the specific application and the characteristics of the compounds being analyzed. Careful consideration of these factors will ensure the selection of the most effective method for achieving optimal separation and analysis.
Latest Posts
Latest Posts
-
The Abdominopelvic And Thoracic Cavities Are Subdivisions Of The
Mar 24, 2025
-
How To Find The Least Squares Solution
Mar 24, 2025
-
Reactants And Products Of The Calvin Cycle
Mar 24, 2025
-
Electric Field For A Line Of Charge
Mar 24, 2025
-
What Is Fusion In Chemistry Phase Changes
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Normal Phase Vs Reverse Phase Hplc . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
