What Is Fusion In Chemistry Phase Changes
Muz Play
Mar 24, 2025 · 6 min read
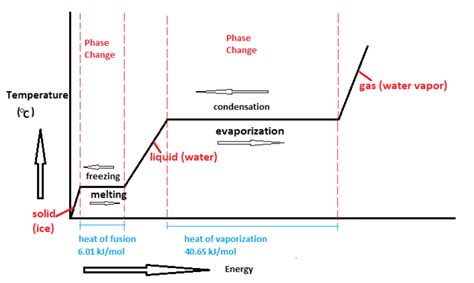
Table of Contents
What is Fusion in Chemistry Phase Changes? A Comprehensive Guide
Fusion, also known as melting, is a fundamental phase transition in chemistry where a substance changes from a solid state to a liquid state. This transformation is driven by the absorption of energy, which weakens the intermolecular forces holding the solid's structure together, allowing the particles to move more freely. Understanding fusion is crucial in various scientific fields, including materials science, chemistry, and physics, as it impacts the properties and behavior of countless substances.
Understanding Phase Transitions and the Role of Energy
Before delving into the specifics of fusion, let's establish a broader understanding of phase transitions. Matter exists in various phases, including solid, liquid, gas, and plasma. These phases are characterized by different arrangements of particles and the strength of the intermolecular forces between them. Phase transitions involve a change in the state of matter, driven by changes in temperature and/or pressure. These transitions are generally accompanied by changes in energy:
-
Endothermic Transitions: These transitions absorb energy from the surroundings. Melting (fusion) is a prime example, as it requires energy input to overcome the intermolecular forces holding the solid together. Other endothermic transitions include vaporization (liquid to gas) and sublimation (solid to gas).
-
Exothermic Transitions: These transitions release energy to the surroundings. Freezing (liquid to solid) is an exothermic transition, releasing energy as the particles slow down and form stronger intermolecular bonds. Other exothermic transitions include condensation (gas to liquid) and deposition (gas to solid).
The Role of Intermolecular Forces
The strength of intermolecular forces significantly influences the melting point of a substance. Stronger intermolecular forces require more energy to overcome, resulting in higher melting points. Conversely, weaker forces lead to lower melting points. The types of intermolecular forces include:
-
London Dispersion Forces (LDFs): These are the weakest intermolecular forces, present in all molecules, and arise from temporary fluctuations in electron distribution.
-
Dipole-Dipole Interactions: These forces occur between polar molecules with permanent dipoles.
-
Hydrogen Bonding: A special type of dipole-dipole interaction involving hydrogen atoms bonded to highly electronegative atoms (like oxygen, nitrogen, or fluorine). Hydrogen bonds are relatively strong.
-
Ionic Bonds: These are strong electrostatic attractions between oppositely charged ions in ionic compounds.
The Fusion Process: A Microscopic Perspective
At the microscopic level, the fusion process involves a disruption of the ordered arrangement of particles in a solid. In a solid, particles are tightly packed in a regular lattice structure, held together by strong intermolecular forces. As energy is added (typically in the form of heat), the particles gain kinetic energy, causing them to vibrate more vigorously.
At the melting point, the kinetic energy of the particles becomes sufficient to overcome the intermolecular forces holding the lattice structure together. The particles break free from their fixed positions and begin to move more randomly, transitioning from the rigid structure of a solid to the more fluid state of a liquid. This doesn't necessarily mean the intermolecular forces vanish; they merely become weaker and less restrictive.
Factors Affecting the Melting Point
Several factors can influence the melting point of a substance:
-
Intermolecular Forces: As previously discussed, stronger intermolecular forces lead to higher melting points. Substances with strong hydrogen bonds, for example, typically have higher melting points than those with only London dispersion forces.
-
Molecular Weight: Larger molecules generally have higher melting points due to increased London dispersion forces. The larger surface area provides more opportunities for temporary dipole interactions.
-
Molecular Structure: The shape and structure of a molecule influence the packing efficiency in the solid state. Molecules that pack more efficiently tend to have higher melting points due to stronger intermolecular interactions.
-
Pressure: Increased pressure generally increases the melting point. This is because higher pressure forces the molecules closer together, strengthening intermolecular forces and requiring more energy to overcome them. However, there are exceptions to this rule, particularly for substances like water, where the solid phase (ice) is less dense than the liquid phase.
-
Impurities: The presence of impurities can lower the melting point of a substance. Impurities disrupt the regular lattice structure of the solid, making it easier for the particles to transition to the liquid phase. This phenomenon is utilized in techniques like determining the purity of a substance through melting point depression.
Fusion in Different Substances
The fusion process varies depending on the type of substance. For example:
-
Crystalline Solids: These solids have a highly ordered arrangement of particles in a regular lattice structure. Their melting points are typically sharp and well-defined. The melting process usually occurs at a constant temperature, as the added energy goes into breaking the lattice structure.
-
Amorphous Solids: These solids lack a well-defined lattice structure, with particles arranged randomly. They don't have a sharp melting point but rather soften over a range of temperatures. Examples include glass and plastics.
Applications of Fusion
Understanding and controlling the fusion process is critical in many applications:
-
Materials Science: The melting point is a key property considered when selecting materials for specific applications. For example, materials with high melting points are required for high-temperature applications.
-
Metallurgy: Melting is fundamental to many metallurgical processes, including the production of alloys and the casting of metal parts.
-
Chemistry: The melting point is often used to identify and characterize substances. It is also important in various chemical processes such as recrystallization.
-
Food Science: The melting point of fats and oils is crucial in food preparation and storage.
-
Environmental Science: The melting of glaciers and polar ice caps is a significant indicator of climate change.
Fusion and Enthalpy of Fusion
The enthalpy of fusion (ΔHfus), also known as the heat of fusion, is the amount of energy required to melt one mole of a substance at its melting point. It's a measure of the strength of the intermolecular forces in the solid. A higher enthalpy of fusion indicates stronger intermolecular forces. This value is an important thermodynamic property that provides insight into the energy changes associated with the phase transition.
The enthalpy of fusion can be calculated using the following equation:
ΔHfus = q / n
Where:
- ΔHfus is the enthalpy of fusion (in J/mol or kJ/mol)
- q is the heat absorbed (in J or kJ)
- n is the number of moles of the substance
Fusion Curves and Phase Diagrams
Phase diagrams graphically represent the conditions of temperature and pressure at which different phases of a substance are stable. These diagrams typically include a fusion curve, which shows the relationship between temperature and pressure at the melting point. The fusion curve indicates that the melting point usually increases with increasing pressure. However, as mentioned earlier, water is a notable exception to this rule.
Conclusion: A Deeper Understanding of Fusion
Fusion, the transition from solid to liquid, is a crucial phase change with far-reaching implications across various scientific disciplines. By understanding the factors influencing melting points, the energy changes involved, and the microscopic processes underlying fusion, we gain a deeper appreciation of the behavior of matter and its application in diverse fields. From material science to environmental studies, the principles of fusion are fundamental to our understanding of the world around us and our ability to harness the properties of matter for technological advancement. Further exploration into this topic reveals a rich and complex interplay between energy, intermolecular forces, and the structure of matter.
Latest Posts
Latest Posts
-
An Irreversible Inhibitor Is One That
Mar 25, 2025
-
When Elements Combine To Form Compounds
Mar 25, 2025
-
Describe The Sampling Distribution Of P Hat
Mar 25, 2025
-
How To Determine State Of Matter In Chemical Equation
Mar 25, 2025
-
Resistors In Series And Parallel Calculator
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is Fusion In Chemistry Phase Changes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
