Nucleotides Are The Building Blocks Of
Muz Play
Mar 21, 2025 · 7 min read
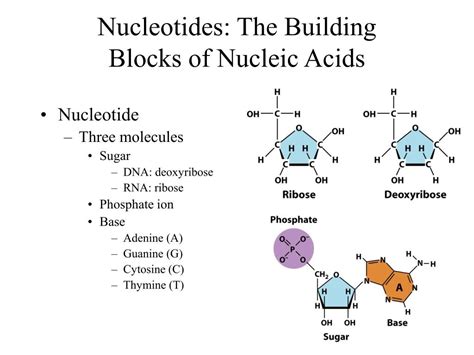
Table of Contents
Nucleotides: The Building Blocks of Life
Nucleotides are the fundamental building blocks of nucleic acids, the vital biopolymers that underpin life as we know it. These remarkable molecules are not just structural components; they play multifaceted roles in various cellular processes, extending far beyond their contribution to DNA and RNA. Understanding nucleotides is crucial to comprehending the intricacies of genetics, heredity, and the very essence of life itself.
The Structure of a Nucleotide: A Molecular Trio
A nucleotide is a molecular assembly comprising three key components:
1. A Nitrogenous Base: The Information Carrier
The nitrogenous base forms the heart of the nucleotide's information-carrying capacity. There are five primary nitrogenous bases found in biological systems:
- Adenine (A): A purine base characterized by a double-ring structure.
- Guanine (G): Another purine base, also possessing a double-ring structure.
- Cytosine (C): A pyrimidine base with a single-ring structure.
- Thymine (T): A pyrimidine base found primarily in DNA.
- Uracil (U): A pyrimidine base that replaces thymine in RNA.
The specific sequence of these bases along the nucleic acid chain encodes the genetic information that directs the synthesis of proteins and regulates gene expression. The pairing between these bases (A with T or U, and G with C) via hydrogen bonds is crucial for the double helix structure of DNA and various RNA secondary structures. This base pairing is fundamental to DNA replication, transcription, and translation – the processes responsible for heredity and protein synthesis.
2. A Pentose Sugar: The Structural Backbone
The second component is a five-carbon sugar, or pentose. There are two types of pentose sugars found in nucleotides:
- Ribose: Found in ribonucleotides, the building blocks of RNA.
- Deoxyribose: Found in deoxyribonucleotides, the building blocks of DNA. The key difference lies in the absence of a hydroxyl (-OH) group on the 2' carbon of deoxyribose. This seemingly minor difference has profound implications for the stability and function of DNA compared to RNA. DNA, being more stable, is better suited for long-term storage of genetic information.
The pentose sugar provides the structural framework to which the nitrogenous base and phosphate group attach, forming the nucleotide's backbone. The specific arrangement of atoms in the sugar ring dictates the overall geometry and three-dimensional structure of the nucleic acid.
3. A Phosphate Group: The Energy Currency and Linkage
The phosphate group, typically a triphosphate in free nucleotides (like ATP), is the energy-rich component of the nucleotide. It connects to the 5' carbon of the pentose sugar. This phosphate group plays several critical roles:
- Energy Transfer: Adenosine triphosphate (ATP), a crucial nucleotide, is the primary energy currency of the cell. The hydrolysis (breaking) of the phosphate bonds releases energy that drives numerous cellular processes.
- Nucleic Acid Linkage: In the formation of nucleic acids (DNA and RNA), the phosphate group acts as a bridge, linking the 5' carbon of one nucleotide to the 3' carbon of the next, forming the characteristic phosphodiester bond. This creates the sugar-phosphate backbone of the polynucleotide chain.
The phosphate group's charge also contributes to the overall negative charge of DNA and RNA, affecting their interactions with proteins and other molecules within the cell.
Nucleotides Beyond DNA and RNA: A Diverse Cast of Characters
While nucleotides are primarily known for their role in forming DNA and RNA, their functions extend far beyond these nucleic acids. They are involved in a wide array of essential cellular processes:
1. Energy Metabolism: The Powerhouses
ATP, the most prominent example, is the primary energy carrier in cells. Its hydrolysis releases energy that fuels countless reactions, including muscle contraction, active transport across cell membranes, and biosynthesis of macromolecules. Other nucleoside triphosphates like GTP, CTP, and UTP also participate in energy transfer and metabolic pathways. These molecules are integral parts of various metabolic cycles and enzyme-catalyzed reactions.
2. Signal Transduction: Cellular Communication
Cyclic nucleotides, such as cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), act as crucial second messengers in signal transduction pathways. These cyclic nucleotides are generated from ATP and GTP, respectively, through the action of specific enzymes (adenylyl cyclase and guanylyl cyclase). They relay signals from cell surface receptors to intracellular targets, initiating a cascade of events that regulate diverse cellular functions, including gene expression, metabolism, and cell growth.
3. Enzyme Cofactors: Catalytic Helpers
Some nucleotides function as essential cofactors for enzymes, assisting in their catalytic activities. Nicotinamide adenine dinucleotide (NAD+), nicotinamide adenine dinucleotide phosphate (NADP+), and flavin adenine dinucleotide (FAD) are prime examples. These coenzymes participate in redox reactions (electron transfer), playing critical roles in metabolism, particularly in energy production and biosynthesis.
4. Cellular Regulation: The Control Center
Nucleotides are involved in regulating various cellular processes through their interaction with proteins and other molecules. For example, they can influence the activity of enzymes, modulate gene expression, and participate in DNA repair mechanisms. The interplay between nucleotides and other cellular components is crucial for maintaining cellular homeostasis and responding to environmental changes.
The Significance of Nucleotide Structure and Function: A Biological Symphony
The precise structure of nucleotides is intrinsically linked to their diverse functions. The specific nitrogenous base determines the genetic code, the pentose sugar influences the stability and type of nucleic acid, and the phosphate group provides energy and links nucleotides together. The subtle differences between ribose and deoxyribose, for instance, explain why RNA is more prone to hydrolysis than DNA – a crucial distinction for the long-term storage of genetic information. Similarly, the high energy phosphate bonds in ATP are precisely engineered to efficiently release energy when needed, fueling the cell's activities.
Nucleotide Synthesis and Degradation: A Dynamic Equilibrium
Cells constantly synthesize and degrade nucleotides to maintain the appropriate levels for their various functions. Nucleotide biosynthesis involves complex pathways that require energy and specific enzymes. These pathways are tightly regulated to prevent excessive accumulation of nucleotides, which can be harmful to the cell. Nucleotide degradation, on the other hand, involves a series of enzymatic steps that break down nucleotides into their constituent components, which can then be recycled or excreted. This dynamic equilibrium ensures that the cell has the necessary building blocks and energy sources available when needed, while preventing the accumulation of potentially toxic substances.
Nucleotide Analogues and Their Applications: Mimicking Nature's Building Blocks
Nucleotide analogues are synthetic molecules that resemble natural nucleotides but have slight structural modifications. These analogues have found extensive applications in various fields:
- Antiviral and Anticancer Drugs: Many antiviral and anticancer drugs are nucleotide analogues. They interfere with viral replication or tumor cell growth by inhibiting enzymes involved in DNA or RNA synthesis. The structural similarity to natural nucleotides allows them to incorporate into DNA or RNA, causing chain termination or disrupting the function of the nucleic acid.
- Diagnostic Tools: Fluorescently labeled nucleotides are used extensively in molecular biology techniques such as PCR (polymerase chain reaction) and DNA sequencing. They enable scientists to visualize and analyze DNA and RNA molecules.
- Research Tools: Nucleotide analogues are invaluable tools in molecular biology research. They are used to study DNA replication, RNA transcription, and other cellular processes. By modifying the structure of a nucleotide, researchers can gain insights into the mechanisms of these essential biological processes.
Conclusion: The Foundation of Life
In conclusion, nucleotides are far more than just the building blocks of DNA and RNA. Their crucial roles in energy metabolism, signal transduction, enzyme cofactor function, and cellular regulation make them fundamental components of all life forms. Understanding the structure, function, synthesis, and degradation of nucleotides is essential for comprehending the complex processes that sustain life, opening doors to advancements in medicine, biotechnology, and our fundamental understanding of the biological world. Further research continues to uncover new roles and functionalities of these remarkable molecules, emphasizing their continued importance in the ongoing quest to unravel the mysteries of life itself. The intricate interplay of these seemingly simple molecules forms the beautiful and complex symphony of life.
Latest Posts
Latest Posts
-
A Larger Nucleus Splits Apart Making 2 Smaller Ones
Mar 27, 2025
-
What Is A Metaparadigm Of Nursing
Mar 27, 2025
-
Song Lyrics Ode To Billy Joe
Mar 27, 2025
-
What Happens During The Reduction Stage Of The Calvin Cycle
Mar 27, 2025
-
Is Solid To Liquid Endothermic Or Exothermic
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Nucleotides Are The Building Blocks Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
